1. Background
Hepatobiliary fascioliasis (HF) is a parasitic liver disease caused by the trematodes Fasciola hepatica and Fasciola gigantica. The HF has been reported worldwide, with significantly high prevalence rates in South America (9.0%), Africa (4.7%), and Asia (2.0%) (1). Fascioliasis remains a significant public health issue due to its increasing incidence in recent years (2). Humans become infected by consuming water or water-related vegetables contaminated with F. hepatica metacercariae (3).
The course of fascioliasis comprises four periods: Incubation, hepatic (acute or invasive), latent, and biliary (chronic or obstructive) (2). The incubation period, during which initial symptoms appear following the ingestion of metacercariae, lasts from a few days to two to three months (2). After oral ingestion, the parasite reaches the liver through the peritoneal cavity by passing through the intestinal wall (2, 4). The hepatic period begins with the slow migration of the parasite in the liver parenchyma and lasts several months (2, 4). During this period, mature parasites digest hepatocytes, forming tunnels and cavitation (2, 4). This is followed by a latent period, during which the parasites mature and oviposition begins. Asymptomatic individuals in the latent period, which can last for months or years, are often detected during family screening after a patient's diagnosis (5). The parasite then settles into the biliary tract, initiating the biliary period, which can persist for years (2). The hepatic and biliary periods, where most diagnoses occur, are the most critical (2).
For diagnosing HF, computed tomography (CT) and magnetic resonance imaging are preferred imaging methods during the hepatic period, while ultrasonography is particularly used during the biliary period (6-9). Diagnoses should be confirmed with serological and parasitic tests (6, 7). Triclabendazole (TCZ) is effective across all periods of HF, with a cure rate of 90% (10).
The recommended follow-up criteria for evaluating treatment success typically include results from short-term follow-up studies involving patients in the biliary period. These studies recommend monitoring clinical response, eosinophil correction, ultrasound improvement, and fecal parasite negativity (11). There is a lack of data on the long-term serology and radiology results of patients in the hepatic period. These long-term follow-up results may aid in interpreting serological and radiological findings during follow-up, particularly in regions where the infection is less prevalent, and may help avoid unnecessary investigations and treatment approaches (12).
2. Objectives
This study aimed to evaluate the long-term laboratory findings, antibody levels, and CT features following the treatment of patients with HF.
3. Methods
In this retrospective study, a total of 25 patients with HF infection were identified at the Medical Faculty Hospital in southeastern Turkey from March 2009 to December 2015. The diagnostic criteria for patients with HF infection in the hepatic period included: (1) The presence of characteristic signs of HF on abdominal CT examination; (2) specific positivity for FH in the ELISA; and/or (3) the appearance of FH eggs in the stool during examination. Diagnoses during the biliary period were made by the appearance of FH eggs in the stool or the detection of live parasites during the endoscopic retrograde cholangiography procedure. All patients received two doses of 10 - 12 mg/kg TCZ (Egaten, Novartis, Basel, Switzerland). Complete clinical and laboratory improvement was achieved in all patients three months after therapy.
Patients who had malignancy, pregnancy, transplant recipients, chronic liver disease, chronic kidney disease, hematological disease, and those using corticosteroids and other immunosuppressive drugs, and who had no abdominal CT and serology at both admission and follow-up were not included in the study. Pre-treatment and follow-up scans as described in previous studies (13).
3.1. Anti-Fasciola Ab Serology
A 3-cc blood serum sample were taken from each of the patients. The blood serums were stored at -20°C until their analysis. The excretory/secretory antigens have been used for immunodiagnosis of fascioliasis in the ELISA kit (DRG International Inc., USA), and the absorbance value was calculated according to the following formula: The cut-off value of the kit was 10; patient sera with a > 11.0 DRG units = DU/mL [DU = patient absorbance value × 10 / cut-off (C1 + C2 / 2)] were determined to be seropositive. For patients with results within the cut off value: (1) ELISA method was repeated after 15 days; and (2) the possibility of cross reactions (hydatid cyst, schistosomiasis and toxocariasis) was taken into consideration.
3.2. Detection of FH Eggs in the Stool
A hazelnut-sized stool sample was collected from each patient to investigate the presence of eggs. The stool samples were examined on the same day. Eggs were identified using the native-Lugol method and the sedimentation method with formaldehyde. In cases where no eggs were observed on the first day, stool microscopy was performed over three consecutive days (14).
3.3. Ethical Approval
This study was approved by the Ethics Committee of the Dicle University School of Medicine (ethics approval number: 231/2023, date: September 13, 2023). The clinical, laboratory, and radiological features of the patients were evaluated in accordance with the principles of the 2008 Helsinki Declaration.
3.4. Statistical Analyses
Data analysis was performed using SPSS (version 21.0). Inter-group comparisons were conducted using the Student’s t-test. Continuous variables were compared with a paired sample t-test. The McNemar test was used to compare the proportions of categorical variables. Groups with categorical variables were compared using the Pearson’s chi-square test. Pearson/Spearman correlation analysis was performed to assess the relationship between variables. A P-value of less than 0.05 was considered statistically significant.
4. Results
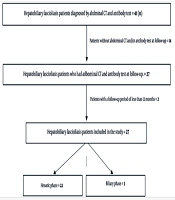
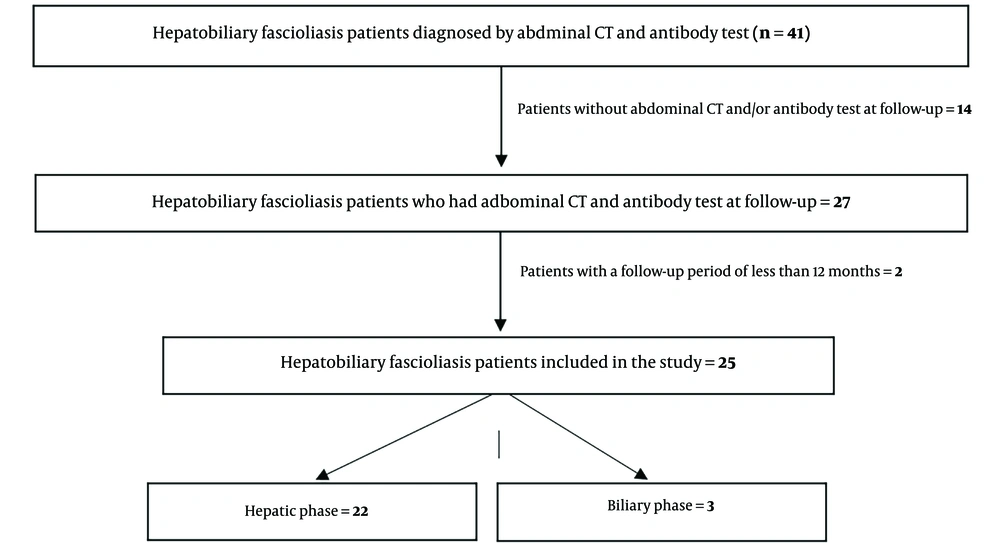
Forty-one patients with HF were identified. Fourteen patients did not have follow-up abdominal CT and/or antibody tests, and two patients had a follow-up period shorter than 12 months; therefore, they were excluded from the study (Figure 1). A total of 25 patients were included in the study. Of these, 20 were female, with a mean age of 41 years (age range: 21 to 63 years). Twenty-two patients were diagnosed during the hepatic period, and three were diagnosed during the biliary period. The follow-up duration for patients ranged from 14 to 58 months, with a mean follow-up duration of 43.0 ± 10.3 months (± SD).
Laboratory results before and after treatment are presented in Table 1. Significant changes were primarily observed in eosinophil counts and Fasciola antibody levels, as well as in liver function test values (ALT, AST, GGT, and ALP), CRP levels, and erythrocyte sedimentation rates (Table 1). The eosinophil count decreased over time, with mean counts declining to 827/mm3 (n = 10), 458/mm3 (n = 19), and 219/mm3 (n = 12) at the fourth, twelfth, and twenty-fourth weeks, respectively.
| Variables and Normal Range | Pre-treatment | Follow-up | Difference (%) | P-Value b |
|---|---|---|---|---|
| AST (10 - 35 U/L) | 30 ± 19 | 18 ± 6 | -26.3 ± 7.0 | 0.002 |
| ALT (10 - 40 U/L) | 45 ± 41 | 19 ± 16 | -36.2 ± 9.7 | 0.002 |
| GGT (5 - 55 U/L) | 105 ± 117 | 27 ± 32 | -55.4 ± 6.4 | 0.003 |
| ALP (40 - 150 U/L) | 145 ± 105 | 70 ± 21 | -36.9 ± 5.9 | 0.001 |
| Total bilirubin (0.2 - 1.0 mg/dL) | 0.60 ± 0.36 | 0.66 ± 0.33 | 37.6 ± 15.6 | > 0.05 |
| WBC (4.600 - 10.200 n/mm3) | 9270 ± 3590 | 7.920 ± 2.130 | -2.4 ± 8.6 | > 0.05 |
| Hemoglobin (12.2 - 18.1 g/dL) | 12.1 ± 2.2 | 13.5 ± 2.0 | 14.1 ± 4.5 | 0.002 |
| Eosinophil (0 - 400 n/mm3) | 2027 ± 2197 | 178 ± 152 | -70.4 ± 7.1 | < 0.001 |
| ESR (0 - 15 mm/h) | 32 ± 31 | 12 ± 10 | -31.7 ± 11.8 | 0.002 |
| CRP (0.1 - 1.0 mg/dL) | 2.80 ± 4.5 | 0.4 ± 0.2 | -43.8 ± 9.7 | 0.013 |
| Fasciola Ab (< 11.0 DU/mL) | 24 ± 8 | 14 ± 9 | -41.2 ± 5.1 | < 0.001 |
| Liver measurement (mm) | 178.32 ± 28.98 | 164.64 ± 22.75 | -7.0 ± 1.7 | < 0.001 |
| Spleen measurement (mm) | 119.40 ± 18.38 | 103.20 ± 15.28 | -12.8 ± 2.3 | 0.002 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transpeptidase; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
a Values are expressed as mean ± SD.
b P-value less than 0.05 was considered statistically significant.
Endoscopic retrograde cholangiopancreatography (ERCP) was performed in only 3 (12%) patients due to clinical or laboratory findings indicative of extrahepatic biliary obstruction. The diagnosis of fascioliasis in these patients was confirmed by the extraction of live, mobile Fasciola hepatica from the extrahepatic biliary ducts during ERCP.
The mean Fasciola antibody levels (± SD) before treatment were 24.60 ± 8.71 DU/mL (range: 12 - 46 DU/mL), and after treatment, they were 14.96 ± 9.73 DU/mL (range: 2 - 44 DU/mL). The mean decrease in antibody levels was 41% (range: 4 - 86%), with antibody negativity detected in 10 patients. A positive correlation was found between the change in Fasciola antibody levels and the change ratios of AST (r = 0.408, P = 0.043), ALT (r = 0.528, P = 0.007), and eosinophils (r = 0.428, P = 0.033). No correlation was detected between antibody negativity and other parameters.
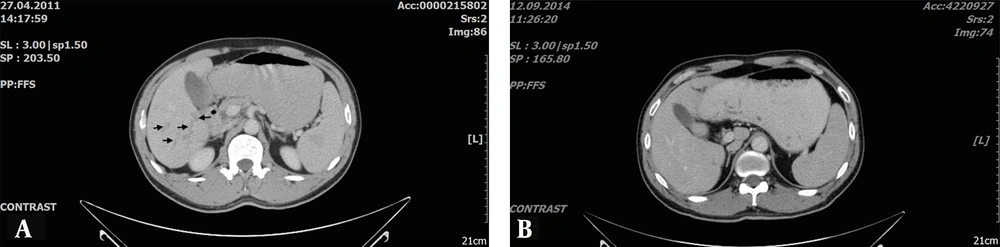
In evaluating the correlation between variables, pre-treatment anti-Fasciola antibody levels were positively correlated with ALP (r = 0.418, P = 0.038), total bilirubin (r = 0.446, P = 0.025), and CRP (r = 0.637, P = 0.001), and negatively correlated with albumin (r = 0.504, P = 0.010). A positive correlation was observed between follow-up anti-Fasciola antibody levels and AST (r = 0.598, P = 0.002). The time between pre-treatment and follow-up CT imaging varied from 12 to 56 months (mean: 37.9 ± 13.5 months). When CT results were evaluated (Table 2), 84% of patients had liver lesions initially, and in 9 patients (36%), liver lesions were completely resolved (Figure 2A - B).
| Radiological Findings | Pre-treatment | Follow-up | P-Value |
|---|---|---|---|
| Liver lesions | 21 (84) | 12 (48) | 0.004 |
| Periportal lymphadenomegaly | 15 (60) | 2 (8) | < 0.001 |
| Hepatomegaly | 12 (48) | 7 (28) | NS |
| Splenomegaly | 7 (28) | 1 (4) | 0.03 |
| Biliary tract lesions | 3 (12) | 0 (0) | NS |
| Intraabdominal free fluid | 3 (12) | 0 (0) | NS |
Abbreviation: NS, not significant.
a Values are expressed as No. (%).
At diagnosis, portal lymphadenopathy was detected in 15 patients (60%); after treatment, only 2 patients had portal lymphadenopathy. Hepatomegaly was initially observed in 12 patients (48%); after follow-up, 7 patients (28%) still had hepatomegaly. Splenomegaly was initially observed in 7 patients (28%); after follow-up, only 1 patient still had splenomegaly. Biliary changes (n = 3) and intra-abdominal free fluid (n = 3) were observed before treatment, and both findings were completely resolved in all patients after treatment. No correlation was detected between radiological improvement and other parameters.
5. Discussion
Fascioliasis remains a significant public health issue due to its increasing incidence in recent years (2). The disease is transmitted through the consumption of contaminated food. Currently, increased travel opportunities, the transportation of plants from endemic to non-endemic regions, and the enhanced use of radiological and serological diagnostic tools may contribute to the rising incidence (13). In non-endemic regions, the radiological findings of fascioliasis may be indistinguishable from other hepatobiliary and bowel diseases, such as malignancy, liver abscess, amebiasis, and hydatid cysts (12, 15-17). Consequently, unnecessary diagnostic and therapeutic interventions may lead to increased mortality and healthcare costs (16, 17).
Data regarding the long-term follow-up of patients in the hepatic phase is limited. Some studies report response evaluation results post-treatment, but they have limitations such as short follow-up periods, a focus on biliary period patients, and the use of ultrasonography in some cases (11, 18, 19). In this study, we demonstrated that both antibody negativity and radiological improvement require a prolonged period in patients in the hepatic phase. Cross-reactions due to the prevalence of parasitic infections in our region may affect negativity ratios, despite antibody reduction in all patients.
Diagnoses for FH infection are generally based on the presence of parasite eggs in the stool, but this method has limitations. It is not diagnostic and may cause delays in diagnoses during the hepatic period (2). In our study, parasite eggs were investigated in the stool of our patients, and only one tested positive. This patient became negative during the third month of treatment and was in the biliary period.
Serology should be utilized to prevent diagnostic delays and unnecessary surgical interventions in patients outside endemic regions (12). In non-endemic areas, patients in the hepatic period are more frequently observed. This can enhance early detection of hepatic fascioliasis, minimizing liver damage and allowing for detection as early as two weeks post-infection, which is approximately 2 - 3 months before eggs appear in the feces (20). Early diagnosis with antibody tests and appropriate treatment can minimize liver damage (20). Therefore, antibody detection is a suitable method for the early diagnosis and management of the disease. The detection of antibodies against antigens of adult FH using ELISA is the most commonly used method for diagnosing HF (14). This method has been shown to have superior diagnostic sensitivity compared to others, such as complement fixation and indirect hemagglutination (11, 20).
In a few studies where parasite negativity in the stool was used as a treatment response criterion, antibody levels were also analyzed (11, 21). These studies reported antibody negativity at the end of the second month to be between 25% and 72.5%. All studies noted a decrease in antibody levels compared to initial values. In our study, a decrease in antibody levels was observed in all patients. Despite prolonged follow-up durations, our antibody negativity ratio was lower than in other studies. The primary factor for this difference may be the acute (hepatic) period in most of our patients. It is known that the cure rate of parasite treatment is higher in patients in the biliary period (21). In 2 of our 3 biliary period patients, antibody negativity developed. Although there was a proportionally significant difference, statistical analyses were not conducted due to the small number of patients.
Another factor affecting our antibody negativity was low specificity due to cross-reactions with other parasites, despite sensitivity for antibody detection at diagnosis (20). We did not pursue further investigations related to intestinal parasitic infections in our patients.
Ultrasound is not a suitable diagnostic tool during the hepatic period due to the lack of well-defined nodules and the heterogeneous structure of the liver (7, 22). While ultrasounds may appear normal, CT scans can reveal numerous clusters of hypo-attenuated nodules, indicative of necrotic cavities and abscesses (7, 22). In the biliary period, ultrasound findings are crucial for diagnosis and follow-up (7, 22).
Studies on the radiological follow-up of HF typically include patients in the biliary period and focus on ultrasonographic findings in the biliary system (18, 19). In a study with a 60-day follow-up, the complete improvement ratio was reported to be about 60%, focusing on biliary tract abnormalities such as biliary dilatation and monitoring parasites in the biliary tract, without specifying liver parenchyma findings like lymphadenopathy and splenomegaly (18, 19).
Kabaalioglu et al. (mean follow-up of 62 months, n = 87 patients) reported liver lesions (90%), lymphadenopathy (52%), biliary abnormalities (45%), splenomegaly (22%), and subhepatic space fluid (5%) (22). Another study (mean follow-up of 25 months, n = 36 patients) reported liver lesions (83%), lymphadenopathy (69%), biliary abnormalities (27%), hepatomegaly (50%), splenomegaly (22%), and intra-abdominal fluid (22%) (13). Pre-treatment findings in our study are similar to both studies, except for biliary changes.
In the follow-up of the first study, splenomegaly and subhepatic space fluid collection were resolved in all patients, and liver lesions, lymphadenopathy, and biliary abnormalities showed significant improvement (22). In the latter study, only subhepatic space fluid collection was resolved in all patients, with marked improvements in liver lesions, hepatomegaly, lymphadenopathy, and biliary abnormalities (13).
In our follow-up results, we observed complete recovery in intra-abdominal fluid and biliary changes, with significant improvements in liver lesions, splenomegaly, and lymphadenopathy at rates of 52%, 96%, and 92%, respectively. The difference between initial and follow-up radiology findings is likely due to the number of patients in the biliary period and the length of the follow-up period. This is primarily because the majority of patients (88%) in our study were in the hepatic period. We observed complete radiological improvements in all of our biliary period patients. High radiological improvement rates in biliary period patients have been reported in the literature (18, 19, 22).
The major limitations of this study include its retrospective design and single-center setting. Additionally, the study included only a few patients in the biliary period. Lastly, the inability to reach the desired number of patients due to various exclusion criteria weakened the study's power.
5.1. Conclusions
To our knowledge, this study is the first to feature a long follow-up duration, including both CT and antibody levels. Based on our findings and existing literature, both antibody negativity and radiological improvement require a prolonged period in hepatic period patients. However, the reduction in antibody levels compared to initial values can be used to evaluate treatment response. Computerized tomography findings should be carefully interpreted in hepatic period patients, as complete recovery may take longer.
Future multicenter, prospective studies that include a sufficient number of patients in both phases and utilize magnetic resonance imaging instead of CT, along with simultaneous ultrasonography, may provide safer and more informative results. This is because the contrast agent used in MRI is not nephrotoxic and does not involve radiation.