1. Background
Hepatitis B virus (HBV) is a hepatophilic DNA virus causing acute and chronic liver failure (1). Hepatitis B virus infection can lead to chronic Hepatitis B, HepatitisHepatitis B cirrhosis, hepatocellular carcinoma, and other related diseases (2). Hepatitis B virus infection is a public health problem worldwide (3). According to epidemiological studies, about 300 million people worldwide are infected with HBV (4), and more than 200 million of them develop chronic diseases caused by HBV infection (5). Chronic HBV infection can lead to liver fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC), eventually resulting in end-stage liver diseases such as liver failure and death, with poor prognosis (6).
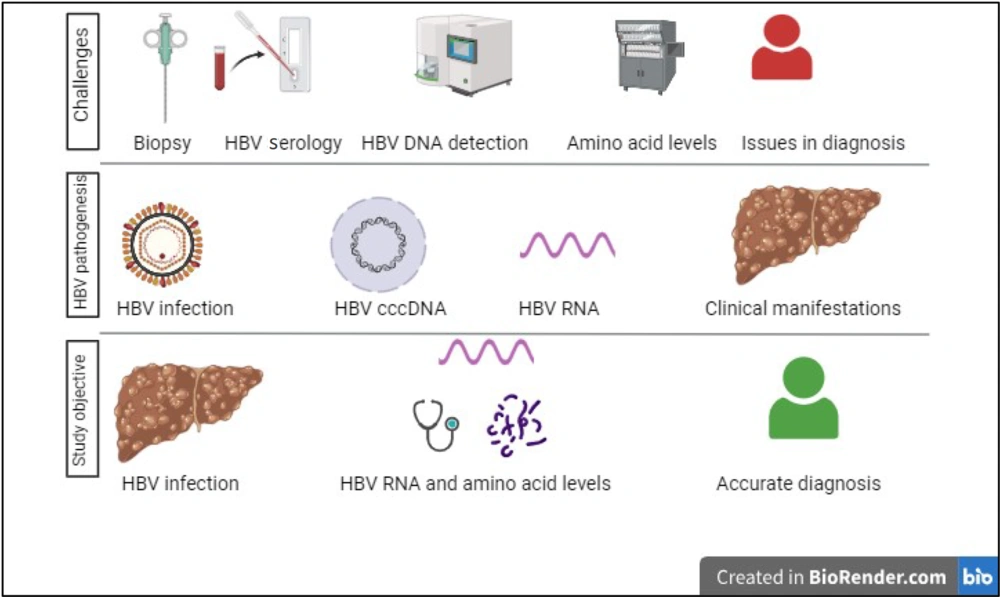
The symptoms of early HBV infection are difficult to detect. Liver biopsy is still considered the gold standard for the diagnosis of liver cirrhosis (7). However, study limitations, such as invasiveness and sampling differences, make liver biopsy not a preferred diagnostic tool (8). Currently, the laboratory detection of HBV infection mainly includes HBV serological tests, HepatitisHepatitis B "two half" tests, liver fibrosis index tests (laminin, type III procollagen peptide, hyaluronic acid, type IV collagen, etc.), and the detection of HBV at the molecular level, such as HBV DNA detection, HBV genotype detection, HBV drug resistance detection, and so on (9-11). However, the aforementioned laboratory tests often require more specificity or cannot rule out the influence of medication. Finding more reliable and convenient laboratory indicators for the diagnosis, treatment, monitoring, and evaluation of chronic HBV infection-related diseases is urgent (Figure 1).
Hepatitis B virus RNA is transcribed from covalently closed circular DNA (cccDNA) (12). As the original synthetic template of HBV pre-genomic RNA (pgRNA) replication, cccDNA is the first genomic replication intermediate that can be detected in the early stage of HBV infection (13). cccDNA can escape the body's immunity and the clearance of antiviral drugs. The content of cccDNA in liver tissue and serum is minimal, making it very difficult to detect. cccDNA serves as the original synthetic template of viral pre-genomic RNA (pgRNA) replication (14); therefore, the detection of HBV RNA levels can replace the detection of cccDNA. Recent studies have shown that HBV RNA was evaluated as a diagnostic marker for chronic Hepatitis B infection (15, 16).
The liver is a critical metabolic site in the human body that is related to amino acid metabolism. Patients with advanced liver diseases such as hepatic encephalopathy have metabolic imbalances in branched-chain and aromatic amino acids (17). Therefore, evaluating the correlation between the metabolic levels of amino acids in chronic HBV infectious diseases and serum HBV RNA levels may help us predict the role and significance of amino acids in the metabolism of chronic HBV infection. Much research has been reported on the use of HBV RNA and microRNA for the diagnosis of liver infection and hepatocellular carcinoma (18, 19).
2. Objectives
This study aimed to investigate the correlation between serum HBV RNA levels and HBV serological test results, amino acid metabolism levels, and clinicopathological parameters in patients at different stages of chronic HBV infection, to find particular, convenient, and accurate laboratory diagnostic indicators for the diagnosis, treatment, and monitoring of chronic HBV infection-related diseases.
3. Methods
Serum samples from 155 patients with chronic HBV infection admitted to the Second Affiliated Hospital of Dalian Medical University from April 2021 to December 2021 were collected. Written informed consent was obtained from each participant according to the guidelines, and the protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University (approval number: 20230728-53). Patient exclusion criteria included other tumors, HIV infection, other liver diseases such as Hepatitis C virus infection, alcoholic liver disease, hereditary liver disease, liver diseases caused by drugs or toxins, biliary obstruction disease, chronic kidney disease or renal failure, severe trauma, pregnancy, and unstable vital signs. All patients were included based on the diagnostic criteria for chronic Hepatitis B of the Chinese Medical Association, 2015 edition (20). Most patients in the chronic Hepatitis B group were currently receiving antiviral therapy, mainly with entecavir or tenofovir, with treatment duration ranging from 1 to 5 months.
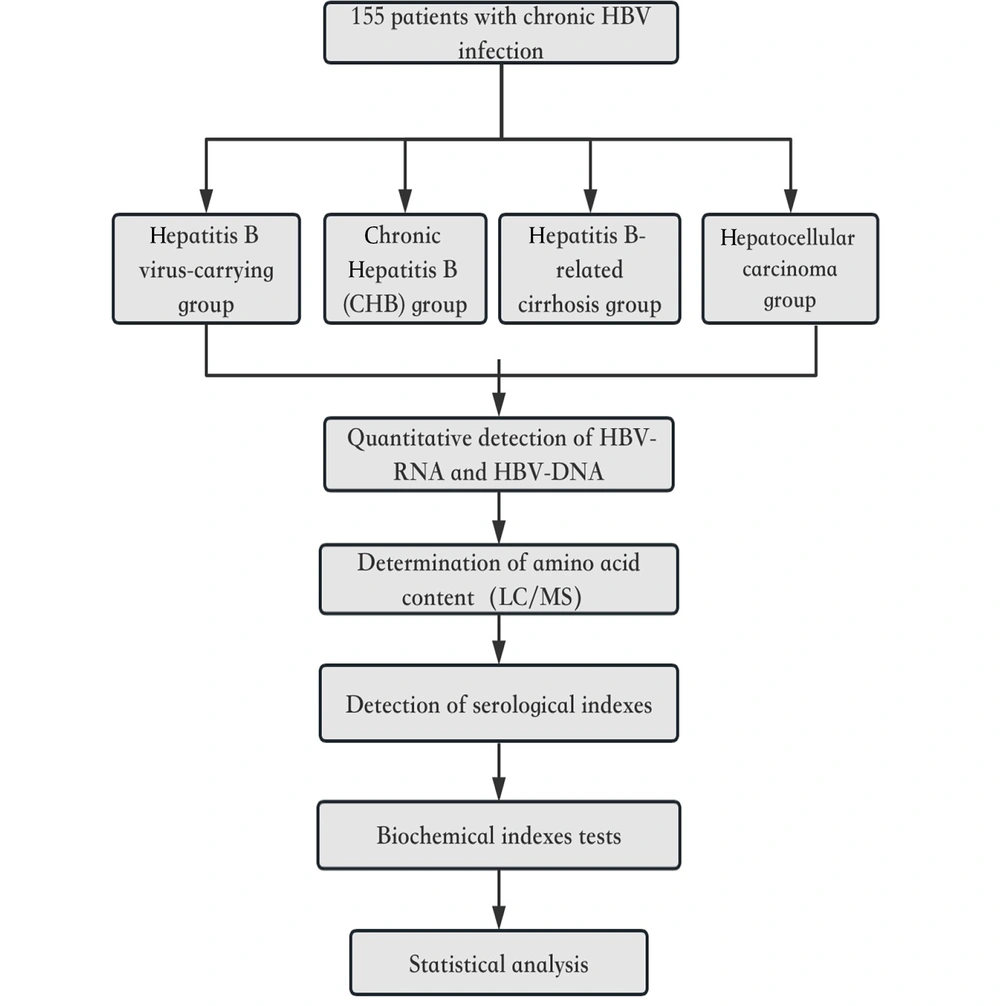
Based on the clinical spectrum of HBV infection, we segregated the patients into four groups: HBV-carrying group, chronic Hepatitis B (CHB) group, Hepatitis B-related cirrhosis group, and hepatocellular carcinoma group (Figure 2). General data, such as age/sex and clinical-related pathological parameters of all cases, were collected simultaneously by the LIS system of the laboratory of the Second Affiliated Hospital of Dalian Medical University.
Biochemical tests, serological tests, and HBV DNA and RNA tests were performed by collecting venous blood samples in the morning on an empty stomach with a biochemical yellow hat tube containing a coagulant. The collection volume was 10 mL. The serum was separated within 2 hours after collection to eliminate interference factors such as hemolysis or chylous blood. The venous blood samples were collected in an enzyme-free centrifuge tube and frozen at -20°C before analysis. The sample (10 mL) was collected in a purple tube containing anticoagulant (EDTA). Samples were stored at 4°C for analysis (21).
3.1. Quantitative Detection of Hepatitis B Virus -RNA
Nucleic acid was extracted with a nucleic acid extraction or purification reagent kit (s10010) from ShengXiang Biotechnology Co., Ltd. The quantitative detection kit of HBV RNA (HBV RNA) from ShengXiang Biotechnology Co., Ltd. (PCR fluorescent probe method) was used for fluorescent RT-PCR amplification. The Shanghai Hongshi slam-96p automatic medical PCR analysis system was used for detection and result analysis. The PCR procedure was as follows: 30 s at 95°C; 5 s at 95°C and 30 s at 60°C for 45 cycles; 5 s at 95°C, 30 s at 60°C, and 15 s at 95℃. All operations were carried out in strict accordance with the manufacturer's instructions. The lower detection limit is 100 IU/mL.
3.2. Quantitative Detection of Hepatitis B Virus -DNA
The HBV nucleic acid assay kit (PCR fluorescent probe method) obtained from ShengXiang Biotechnology Co., Ltd. was used for sample processing and PCR amplification. The Shanghai Hongshi slam-96p automatic medical PCR analysis system was used for detection and result analysis. All operations were carried out in strict accordance with the manufacturer’s instructions. The lower detection limit is 500 IU/mL.
3.3. Determination of Amino Acid Content
Mass spectrometry (LC/MS) was used for detection. The ABI ApI3200 tandem mass spectrometer from American ABI company and LC-20AD high-performance liquid chromatograph (Shimadzu, Japan) were utilized.
3.4. Detection of Serological Indices
HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb were detected using the chemiluminescence method with the architect I2000sr, and the detection was carried out in strict accordance with the standardized operating procedures of the laboratory.
3.5. Biochemical Index Tests
The Siemens Advia2400 automatic biochemical analyzer was used to detect ALT and AST. The reagents were all purchased from Siemens and tested using the rate method in strict accordance with the standardized operating procedures of the laboratory. The reference ranges of ALT and AST are 7 - 40 u/L and 13 - 35 u/L, respectively.
3.6. Statistical Analysis
SPSS 26.0 statistical software was used for data analysis. The measurement data of normal distribution are expressed as mean standard deviation (x̄ ± s). ANOVA was used to compare the four groups. Enumeration data were expressed in [n (%)], and the chi-square test (χ2) was used for comparison among the four groups. Correlation analysis was used to compare the correlation between HBV RNA and various amino acid levels (arginine, asparagine, aspartic acid, glutamine, acetylcarnitine, piperamine, ornithine, citrulline, homocysteine, cysteine, methionine). The factors influencing RNA were analyzed by multivariate logistic regression; ROC curve was used to evaluate the diagnostic value of HBV RNA in the chronic Hepatitis B group, Hepatitis B cirrhosis group, and hepatocellular carcinoma group. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Comparison of General Data and Clinical Indexes Among Four Groups
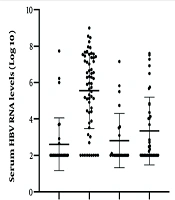
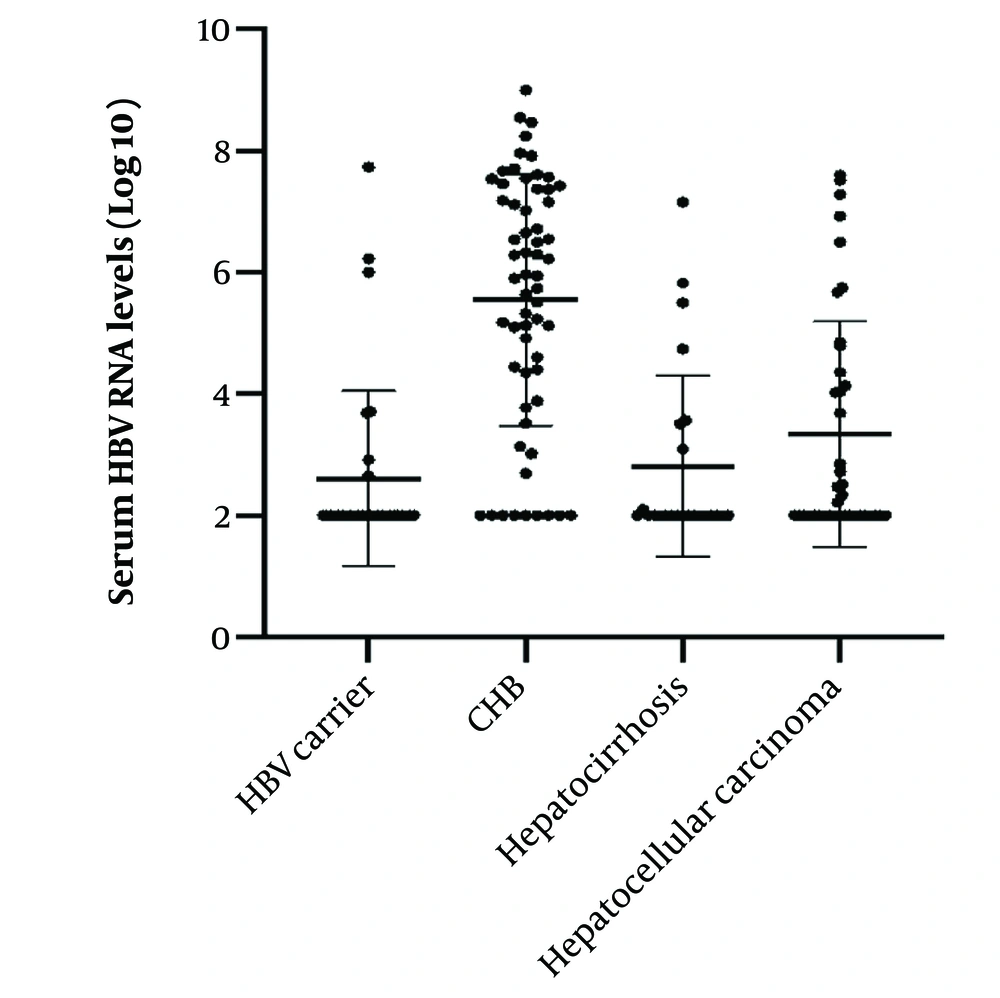
There was no significant difference in age, sex, ALT, AST, and HBeAg levels among the four groups (P > 0.05). There was a statistically significant difference in the HBV DNA, RNA levels, and HBsAg levels among the four groups (P < 0.01) (Table 1). The HBV serum RNA levels of patients with chronic Hepatitis B infections were significantly higher than those of Hepatitis B carriers, liver cirrhosis patients, and hepatocellular carcinoma patients (Figure 3). We have not evaluated the impact of patient medication on the different parameters as some patient records lacked current or previous medication information before enrolling in the study.
| Index | Hepatitis B Carriers (n = 31) | CHB (n = 61) | Cirrhosis (n = 24) | Hepatocellular Carcinoma (n = 39) | F/x2 | P-Value |
|---|---|---|---|---|---|---|
| Age (y) | 53.03 ± 13.20 | 52.07 ± 13.30 | 56.17 ± 10.51 | 56.49 ± 10.47 | 1.236 | 0.394 |
| Gender | 5.017 | 0.171 | ||||
| Male | 19 (61.2) | 41 (67.2) | 20 (83.3) | 31 (79.4) | ||
| Female | 12 (38.7) | 20 (32.7) | 4 (16.6) | 8 (20.5) | ||
| ALT, U/L | 44.59 ± 91.68 | 97.30 ± 196.90 | 102.75 ± 170.04 | 43.34 ± 38.93 | 1.441 | 0.233 |
| AST, U/L | 46.97 ± 109.49 | 69.68 ± 134.18 | 105.54 ± 156.63 | 46.93 ± 34.11 | 1.619 | 0.187 |
| HBV DNA, log10 IU/mL | 3.44 ± 1.32 | 5.12 ± 2.13 | 3.23 ± 1.31 | 3.94 ± 1.71 | 10.041 | < 0.01 |
| HBV RNA, log10 IU/mL | 2.61 ± 1.44 | 5.55 ± 2.06 | 2.81 ± 1.49 | 3.34 ± 1.86 | 25.912 | < 0.01 |
| HBsAg, log10 IU/mL | 2.81 ± 1.31 | 3.68 ± 0.71 | 2.75 ± 1.33 | 3.38 ± 1.08 | 7.708 | < 0.01 |
| HBeAg (COI) | 368.53 ± 1703.46 | 566.82 ± 656.76 | 2.61 ± 8.04 | 343.36 ± 1228.25 | 0.191 | 1.602 |
Comparison of General Data and Clinical Indices of the Four Groups of Patients a
4.2. Correlation Between HBV RNA Levels and Amino Acids in Patients with Chronic Hepatitis B-associated Liver Diseases
Multiple linear regression analysis of the HBV RNA levels in patients with chronic Hepatitis B-related liver diseases with the metabolic levels of various amino acids (arginine, asparagine, aspartic acid, glutamine, acetylcarnitine, piperamine, ornithine, citrulline, homocysteine, cysteine, methionine) showed a significant correlation with methionine levels (P < 0.05). There was no significant correlation between the levels of aspartic acid, glutamine, acetylcarnitine, piperamine, ornithine, citrulline, homocysteine, cysteine, and HBV RNA levels (Table 2).
| Variables | Arg | Asn | Gln | ACL | Piperamide | Orn | CCP | Hcy | Cys | Met |
|---|---|---|---|---|---|---|---|---|---|---|
| x ̅ ± s | 11.81 ± 11.01 | 81.99 ± 23.18 | 10.63 ± 3.97 | 21.84 ± 10.91 | 451.49 ± 178.58 | 40.24 ± 20.43 | 26.01 ± 9.63 | 13.67 ± 11.61 | 287.21 ± 55.51 | 27.92 ± 16.21 |
| Persons’ correlation coefficient | 0.076 | 0.032 | 0.092 | 0.185 | 0.103 | 0.165 | -0.030 | -0.111 | -0.151 | 0.297 |
| P -value | 0.505 | 0.782 | 0.422 | 0.102 | 0.366 | 0.145 | 0.794 | 0.363 | 0.231 | 0.014 a |
Correlation Analysis Between HBV RNA Levels and Amino Acid Metabolism Levels
4.3. Multivariate Logistic Regression Analysis of Amino Acids Affecting HBV RNA Levels in Patients with Chronic Hepatitis B-associated Liver Diseases
The dependent variable was HBV RNA (HBV RNA ≤ 100 IU/mL: HBV RNA negative group = 0; HBV RNA > 100 IU/mL: HBV RNA positive group = 1; 100 IU/mL is the lower limit of HBV RNA detection). Patients were included in the regression analysis after variable assignment. Multivariate logistic regression analysis of HBV RNA levels in patients showed that HBV DNA levels were an independent risk factor for HBV RNA levels (Table 3). Multivariate logistic regression analysis of amino acid types affecting HBV RNA levels showed that arginine, asparagine, aspartic acid, glutamine, acetylcarnitine, piperamine, ornithine, citrulline, homocysteine, cysteine, and methionine were not independent risk factors for HBV RNA levels (P > 0.05) (Table 4).
| HBV RNA | β | Standard Error | Wald χ2 | P | OR | 95%CI |
|---|---|---|---|---|---|---|
| ALT | -0.007 | 0.006 | 1.232 | 0.267 | 1.618 | 0.981 - 1.005 |
| AST | 0.009 | 0.006 | 2.071 | 0.150 | 2.587 | 0.997 - 1.021 |
| HBsAg | -0.564 | 0.336 | 2.809 | 0.094 | 3.728 | 0.294 - 1.100 |
| HBeAg | 0.000 | 0.000 | 2.895 | 0.089 | 2.999 | 0.999 - 1.000 |
| HBV DNA | -0.940 | 0.243 | 15.001 | 0.000 | 28.277 | 0.243 - 0.629 |
| Groups | -1.168 | 0.603 | 3.754 | 0.053 | 14.874 | 0.095 - 1.014 |
| Gender | -0.449 | 0.566 | 0.630 | 0.427 | 0.637 | 0.210 - 1.936 |
Multivariate Logistic Regression Analysis of HBV RNA Levels
| HBV RNA | β | Standard error | Wald χ2 | P | OR | 95%CI |
|---|---|---|---|---|---|---|
| Arg | 0.053 | 0.046 | 1.337 | 0.248 | 1.506 | 0.964 - 1.154 |
| Asn | -0.005 | 0.013 | 0.118 | 0.731 | 0.119 | 0.970 - 1.022 |
| Gln | 0.045 | 0.093 | 0.229 | 0.632 | 0.231 | 0.871 - 1.256 |
| ACL | -0.39 | 0.064 | 0.377 | 0.539 | 0.372 | 0.848 - 1.090 |
| Piperamide | -0.002 | 0.002 | 1.058 | 0.304 | 1.054 | 0.993 - 1.002 |
| Orn | -0.023 | 0.026 | 0.781 | 0.377 | 0.807 | 0.930 - 1.028 |
| CCP | -0.041 | 0.040 | 1.085 | 0.298 | 1.092 | 0.888 - 1.037 |
| Hcy | 0.022 | 0.050 | 0.189 | 0.663 | 0.230 | 0.927 - 1.126 |
| Cys | 0.006 | 0.007 | 0.624 | 0.429 | 0.627 | 0.991 - 1.020 |
| Met | -0.007 | 0.020 | 0.135 | 0.713 | 0.141 | 0.954-1.033 |
Multivariate Logistic Regression Analysis of Amino Acids Affecting HBV RNA Levels
4.4. ROC Curves of Diagnostic Value of HBV RNA Levels in Chronic Hepatitis B Patients, Hepatitis B Cirrhosis Patients, and Hepatocellular Carcinoma Patients
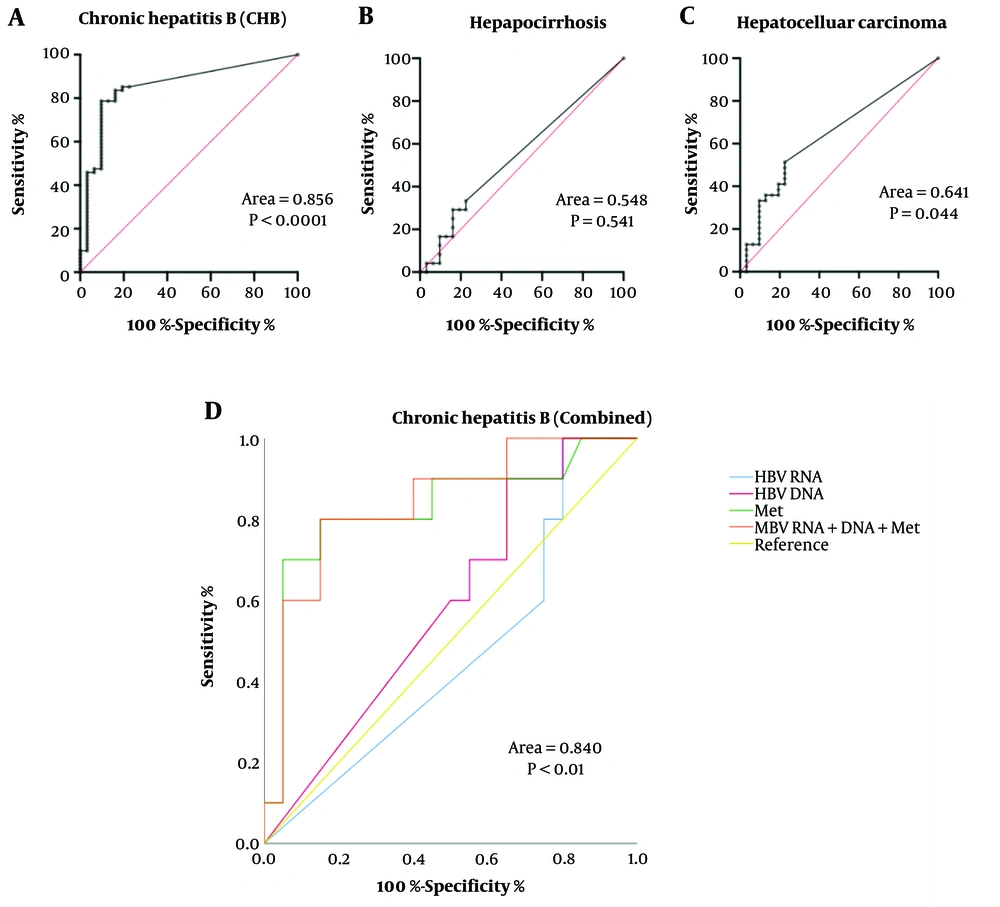
Analysis of the ROC curves and diagnostic value of HBV RNA levels in patients with chronic Hepatitis B, cirrhotic Hepatitis B, and hepatocellular carcinoma (Figure 4) revealed that the AUC of HBV RNA in evaluating chronic Hepatitis B and hepatocellular carcinoma were 0.856 and 0.641, respectively, with a sensitivity of 78.69% and 51.28%, respectively, and a specificity of 90.32% and 77.42%, respectively (P < 0.05). The area under the ROC curve was 0.840 (P < 0.01) when HBV RNA levels, HBV DNA levels, and methionine levels were combined for the diagnosis of chronic Hepatitis B, suggesting that HBV RNA levels and methionine levels have potential value for the diagnosis of chronic infectious diseases of Hepatitis B.
5. Discussion
Hepatitis B virus infection can progress to chronic Hepatitis B, leading to liver fibrosis, cirrhosis, and hepatocellular carcinoma, posing a serious threat to liver health (22). Therefore, timely diagnosis, treatment, and evaluation of HBV infection are essential. In HBV-infected patients, failure to detect serological HBeAg levels and serum HBV DNA levels as indicators of virological response to treatment can increase the risk of virological rebound and disease recurrence, as recommended by AASLD 2015, China/APASL 2015, and EASL 2017 for NAs drug withdrawal (23). Additionally, for HBeAg-negative patients, long-term antiviral treatment results in only a few achieving negative HBsAg conversion, often leading to the need for long-term or lifelong medication (24). Studies have identified the persistence of HBV cccDNA in infected liver nuclei as the primary cause of chronic Hepatitis B (25), suggesting that HBV RNA, as the transcriptional form of cccDNA, could serve as a crucial indicator for the diagnosis, treatment, and monitoring of chronic HBV infectious diseases, as well as guiding safe drug withdrawal (25).
This study aimed to elucidate the correlation between serum HBV RNA levels and HBV serological tests, amino acid metabolism levels, and clinicopathological parameters in patients at different stages of chronic HBV infection. We included four types of patients in this study: Asymptomatic HBV carriers, chronic Hepatitis B patients, Hepatitis B-related cirrhosis patients, and hepatocellular carcinoma patients. The parameters considered included HBV RNA levels, HBV DNA levels, amino acid levels, serological indexes, and common biochemical indexes. There were no significant differences in age and gender among the four groups. Our results indicated that HBV RNA levels in chronic Hepatitis B were significantly higher compared to all other groups considered (P < 0.05), and significant differences were observed in HBV DNA levels and HBsAg levels among the four groups (P < 0.05).
As a crucial metabolic hub in the human body, the liver plays an indispensable role in amino acid metabolism. As early as 1940, changes in plasma amino acid patterns were observed in patients with decompensated liver diseases, and subsequently, the ratio of branched-chain amino acids to aromatic amino acids was studied for diagnosing hepatic encephalopathy. Recent studies have highlighted the value of plasma amino acid concentration as a diagnostic parameter for chronic Hepatitis B and liver fibrosis (26). In this context, Methionine has gained particular importance.
Methionine is essential for maintaining the growth, development, and nitrogen balance of the human body (27). It plays a vital role in synthesizing choline from adrenaline and liver fat. The concentration of methionine and homocysteine has been implicated in the development of Alzheimer's disease, dementia, and changes in brain structure (28). Additionally, methionine and its metabolites are affected by various diseases, including cardiovascular disease and kidney disease, and the methionine cycle plays a pivotal role in a broad spectrum of metabolic diseases (29, 30). Methionine metabolites such as S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), and homocysteine (Hcy) have been suggested to influence the pathological state and the onset and progression of chronic liver diseases (31).
Studies have demonstrated significant correlations between serum SAH and Hcy levels and the SAM/SAH ratio with the degree of liver steatosis (P < 0.001). The increase in serum SAH and Hcy levels and the decrease in the SAM/SAH ratio are independently associated with the development of nonalcoholic fatty liver disease (NAFLD) in middle-aged and elderly Chinese individuals (26). On the other hand, Methionine promotes intrahepatic fat metabolism, offering potential in preventing and treating chronic Hepatitis, liver cirrhosis, fatty liver, and related diseases (32).
Our multivariate logistic regression analysis aimed to uncover any relationship between various amino acids and HBV RNA levels in patients with chronic Hepatitis B-related liver disease. The results indicated that arginine, asparagine, aspartic acid, glutamine, acetylcarnitine, piperamine, ornithine, citrulline, homocysteine, cysteine, and methionine are not independent risk factors for HBV RNA levels (P > 0.05). However, multiple linear regression analysis revealed a significant correlation between HBV RNA levels and methionine levels (P < 0.05). One possible mechanism is that increased methionine metabolites may disrupt the methylation of various substances, including liposome phospholipids (PC) and sterol regulatory element-binding proteins (SREBPs), both known to regulate lipid transport and maintain liver lipid homeostasis (33). Nevertheless, our results showed no significant correlation between HBV RNA levels and homocysteine levels, a methionine metabolite (P = 0.363). Possible reasons could include: (1) Homocysteine, as an independent risk factor for cardiovascular disease, may also be related to the presence of cardiovascular diseases, which might have influenced our results due to the concealed symptoms of cardiovascular diseases at the time (34) of the study; (2) The sensitivity of mass spectrometry used in this study to determine homocysteine content may not be as high as that of ultra-high performance liquid chromatography combined with tandem mass spectrometry (UHPLC-MS/MS); (3) Homocysteine levels are closely associated with folic acid status and vitamin B12 levels (35), warranting further research with a larger sample size.
We also evaluated the diagnostic value of HBV RNA levels in patients with chronic Hepatitis B, Hepatitis B-related cirrhosis, and hepatocellular carcinoma. The area under the ROC curve (AUC) for HBV RNA levels in assessing chronic Hepatitis B and hepatocellular carcinoma was 0.856 and 0.641, respectively. The sensitivity was 78.69% and 51.28%, and the specificity was 90.32% and 77.42% (P < 0.05). These findings suggest that quantitative detection of HBV RNA may aid in evaluating chronic Hepatitis B infectious diseases and can serve as a diagnostic marker for chronic Hepatitis B and hepatocellular carcinoma.
This study has several limitations. While we identified a correlation between serum HBV RNA levels and methionine metabolism, we did not investigate the potential mechanistic pathways or biological mechanisms driving these associations. Exploring the mechanisms underlying the observed correlations can enhance our understanding of the pathophysiology of HBV infection and identify potential therapeutic targets (36). Due to time constraints, the sample size in this study was small, comprising only 155 cases. A future study with a larger sample size and longer observation time for follow-up might provide more in-depth insights and research into whether HBV RNA can serve as one of the indicators for treatment withdrawal. Additionally, the determination of HBV DNA levels may have been influenced by patients taking drugs such as nucleoside analogs or interferon (37), and the potential impact of these drugs on HBV DNA copies cannot be ruled out.
In conclusion, our study revealed that HBV RNA levels in patients with chronic Hepatitis B are significantly higher than those in patients with Hepatitis B, liver cirrhosis, or hepatocellular carcinoma, and they correlate with methionine metabolism levels. Furthermore, we demonstrated a correlation between HBV RNA levels and Hepatitis B infection in patients, suggesting that HBV RNA levels can potentially serve as a valuable predictive efficacy and diagnostic marker for assessing the efficacy of treatment for different stages of chronic HBV infectious diseases.