1. Background
Prevention against infection with hepatitis B virus (HBV) is an important challenging issue in every population, particularly in the developing countries. There are more than 350 million HBV carriers throughout the world (1) and it is estimated that as many as 40% of such people expire due to chronic liver disease, liver cirrhosis or primary hepatocellular carcinoma (2). Although considerable improvements are made in antiviral medications against HBV, still prevention through vaccination is the best measure against spreading the disease (3).
Immune response to vaccination is affected by various factors such as age at which the vaccine is injected, the site of injection, and the number of injected doses. Data shows that three dose vaccination protocol, elicit protective antibody titers (~10 mIU/mL of antibody to hepatitis B surface antigen (HBsAg) in 90% of healthy subjects (4). Hence, approximately 10% of healthy people vaccinated against HBV are estimated to fail in producing a protective level of hepatitis B surface antibody (HBsAb) (3). Accordingly, various methods are implemented to improve immune response in this population such as administration of immune-enhancing medications, increasing the dose of vaccine, changing injection route, etc. (5-8).
Statins or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors that are well known for their cholesterol reduction effects elicit anti-inflammatory effects as well (9). Statins directly inhibit induction of MHC (major histocompatibility complex) class II expression by IFN-γ and thus repress MHC-II-mediated T-cell activation (10). Atorvastatin also inhibits secretion of T helper-1 (Th1) cytokines (interleukin (IL)-2, IL-12) and tumor necrosis factor (TNF-α) and stimulates differentiation of Th0 cells into Th2 cells. Antigen-specific T-cell activation induced by atorvastatin on antigen presenting cells (APC) and T-cells, known as pleiotropic immunomodulatory effects, should be considered for the treatment of either APC or T-cell suppression (11). Short-term atorvastatin treatment enhances specific antibody production in healthy patients following tetanus toxoid vaccination (12).
Considering the possible effects of atorvastatin on further activation of immune system, the current study aimed at evaluating the efficacy and immunogenicity of this drug on subjects not responding to HB vaccination in a prospective randomized trial. The current study also assessed possible immunologic pathways through which this drug might be affecting the immune system by measuring the levels of IL-4, IL-17, interferon (IFN)-γ, and transforming growth factor (TGF)-β cytokines and expression of their corresponding genes including T-Bet, RORc, and GATA3.
2. Methods
2.1. Study Design and Setting
The current study was conducted as a randomized, double-blind, placebo-controlled trial from 2011 to 2012 in Baqiyatallah University of Medical Sciences, Tehran, Iran to determine the immune response effects of short-term atorvastatin administration together with HBV vaccination among non-responder participants.
2.2. Sample Size Calculation
Using the Cohen’s formula for sample size calculation (13), considering α = 0.05 and β = 0.1, the minimum sample size was calculated 26 subjects for each group:
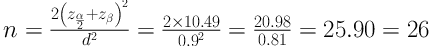
Unfortunately, this number could not be achieved during the recruitment period. Therefore, it was tried to compensate for the small sample population by performing bootstrap in the analyses.
2.3. Participants
Using a convenience sampling method, the study enrolled healthy subjects aged ≥ 18 years who were non-responder to HBV vaccination; that is the ones with anti-HBs titer < 10 mIU/mL two months after receiving the last dose of primary HBV vaccination. The minimum age of 18 years was determined, according to previous studies (12, 14), in order to make sure that the subjects completed their vaccination series and were true non-responders. Participants with a positive test result for serum markers of HBsAg, HBcAb, or HBsAb in the primary evaluations indicating prior or current HBV infection, subjects with chronic liver diseases, underlying diseases affecting the immune system such as HIV, or with a history of taking atorvastatin or immunosuppressive agents such as corticosteroids or methotrexate prior to enrollment were excluded.
2.4. Randomization, Blinding, and Intervention
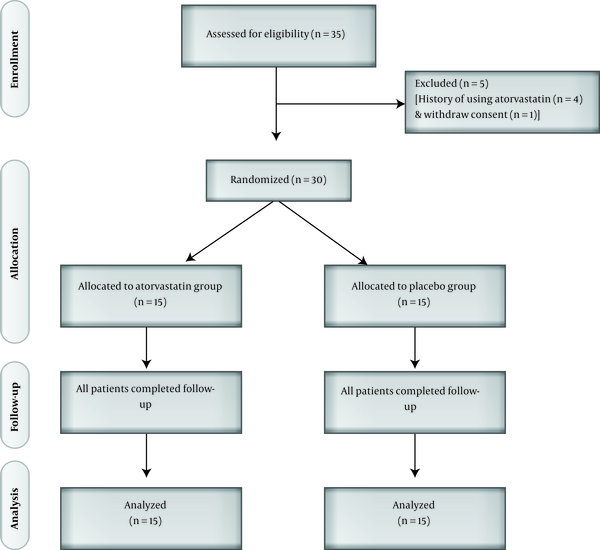
A statistician completely independent of the process of data management and analysis created random sequence generation using a random number table and all participants and investigators were masked to the treatment allocation. Therefore, the participants were randomly assigned in a 1:1 ratio to receive daily dose of either 40 mg atorvastatin (n = 15) or placebo (n = 15) for a 10-day intervention course. Figure 1 shows the flowchart of the study.
Both placebo and atorvastatin were manufactured by Razak Company, Karaj, Iran and had no difference regarding shape, preservative materials, and color except for the active substance of atorvastatin. Considering the pharmacokinetics of atorvastatin and the half-life of HMG-CoA reductase in the body, to make sure that the drug reached a stable serum concentration, HBV vaccine (20 µg/mL) was administered on the 5th day of intervention course, intramuscularly into the deltoid muscle of participants’ left arm using a 20-gauge needle. All vaccines were genetically engineered recombinant hepatitis B (Engerix-B), manufactured by GlaxoSmithKline Biologicals (GSK) in Belgium. They were stored at 2-8°C and maintained within the cold chain through the transportation process.
2.5. Outcomes
Eight weeks after vaccination, 10 mL of blood was collected from each participant, heparinized and sent to a laboratory to assess the level of HBsAg, hepatitis B core antibody HBcAb, hepatitis B surface antibody HBsAb, IL-4,ww IL-17, (IFN)-γ, and TGF-β using the enzyme-linked immunosorbent assay (ELISA) and expression of T-Bet, RORc, and GATA3 genes using the real-time polymerase chain reaction (PCR).
2.6. Stimulation of PBMCs
Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden) was used to separate Peripheral Blood Mononuclear Cells (PBMCs) from the heparinized peripheral blood. Isolated PBMCs were washed in RPMI-1640 medium (Gibco Life Technologies Ltd., Paisley, UK), and then suspended again in the complete culture medium containing RPMI-1640, 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA) and antibiotics including streptomycin (100 µg/mL) and penicillin (100 U/mL) (Biosera, Ringmer, East Sussex, UK).
In the presence of 1 µg/mL anti-CD-28 (Sigma-Aldrich Corporation, St Louis, MO, USA) and 10µg/mL of purified surface antigen of recombinant HBV without any additives (Heberbiotec Co. Cuba), 2 × 106 cell/mL PBMC was seeded in a 24-well sterile culture plate (Nalge Nunc International, Roskilde, Denmark). After 72 hours of incubation at 37°C in humidity plus 5% CO2, culture supernatants were collected and stored at -80°C until used.
2.7. Detecting HBV Markers and Measuring HBsAb Levels
Commercial ELISA kits were used to detect HBsAb (Enzygnost Micro, Boehringer, Germany), HBcAb, and HBsAg (Organon Teknika, The Netherlands). Quantification of HBsAb titer was done by extrapolation from a standard curve constructed using a serum sample provided by the manufacturer with a specific antibody concentration.
2.8. Quantification of Cytokines
Sandwich ELISA was used to measure all of the IL-4, IL-17, IFN-γ, and TGF-β via commercial kits (Biosource International, Camarillo, CA, USA). The assay was optimized for IL-4 according to the manufacturer recommendations by titration of the paired detection and capture antibodies to identify the optimum concentration of both antibodies. In this regard, capture antibodies (1 µg/mL) were coated in polystyrene ELISA plates (Maxisorp, Nunc) and the detection antibodies (biotinylated, 0.4 µg/mL) were used to detect antibodies against this cytokine.
2.9. RNA Isolation and cDNA Synthesis
Following the manufacturer’s instructions, total RNA was isolated from PBMCs (Ribospin, GeneALL, Seoul, Korea). Spectrophotometry was used to monitor RNA concentrations (NanoDrop, Thermo Scientific, Wilmington, DE, USA) and the extracted RNA was assessed for its integrity using gel electrophoresis for the intact 28S and 18S ribosomal RNA. With a first strand complementary DNA (cDNA) synthesis kit (Fermentas, Germany), cDNA was derived from the RNA and stored at -20°C until used.
2.10. Quantitative Real-Time PCR
All PCR amplifications were performed using SYBR Green System (PrimerDesign, Southampton, UK) and assays were conducted in a thermal cycler (RotorGene 6000, Corbett Life Science, Australia). Specific primers for T-bet, GATA3, and RORc as well as β-actin, as the housekeeping gene to normalize mRNA expression levels between different samples were purchased (TAG, Copenhagen, Denmark). The cDNA, synthesized according to the target RNAs using reverse transcriptase enzyme, went through the stages of heating for denaturation and re-synthesis over and over again. After each cycle, the number of genes multiplied and the fluorescent signal received by the device increased. The higher initial number of copies in the sample needed less cycles for its signal intensity to surpass the pre-determined level. For each cDNA, the needed number of cycles was measured and reported as the cycle threshold value (Ct value). The results were also reported as Delta Ct, calculated as the Ct value of the target gene minus the Ct value of β-actin gene.
2.11. Ethical Consideration
The current study was conducted in accordance with the good clinical practice guidelines and the ethical standards of Helsinki declaration. After approval by Institutional Ethics Committee of Baqiyatallah University, the study was registered at the Iranian Registry of clinical trials (code: IRCT201109147556N1) and the USA (NCT01548326). All participants signed informed consent forms before enrollment and they were free to withdraw from the study any time.
2.12. Statistical Analysis
Quantitative and qualitative variables were expressed as mean ± standard deviation (SD) and frequency (percentage), respectively. Chi-square test was used to compare qualitative variables and Student t test to analyze quantitative variables between the study groups. A P-value < 0.05 was considered as statistically significant and SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA) was employed to perform all statistical analyses in the current project.
3. Results
A total of 30 subjects including 25 males (83.3%) and 5 females (16.7%) with the mean age of 29.93 ± 9.41 years were included in the study. General characteristics of the study population are presented in Table 1. As can be observed, there were no significant differences between the participants of the two groups regarding age, gender, body mass index (BMI), cigarette smoking, and primary HBsAb titer, which indicated a successful randomization.
| Characteristics | Total (N = 30) | Atorvastatin Group (N = 15) | Placebo Group (N = 15) | P-Value |
|---|---|---|---|---|
| Age (y), Mean (SD) | 29.7 (9.4) | 29.7 (9.6) | 29.9 (9.6) | 0.99 |
| BMI (kg/m2), Mean (SD) | 25.2 (4.0) | 24.9 (5.0) | 25.5 (3.4) | 0.68 |
| Male gender, N (%) | 25 (83.3) | 13 (86.7) | 12 (80.0) | 0.62 |
| Smoker, N (%) | 5 (16.7) | 4 (26.7) | 1 (6.7) | 0.14 |
| HBsAb titer (mIU/mL), Mean (SD) | 5.7 (1.3) | 5.7 (1.2) | 5.7 (1.5) | 0.93 |
General Characteristics of the Study Participants at Baseline
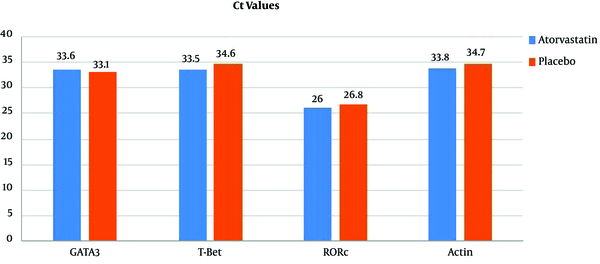
Table 2 presents the seroprotection rate of HBsAb, concentration of measured cytokines, and Ct values for GATA3, T-Bet, RORc, and β-actin at the follow-up time. Delta Ct values are also presented for the three GATA3, T-Bet, and RORc genes, which are normalized Ct values according to that of β-actin’s as the housekeeping gene. As can be observed, HBsAb titers were higher in the atorvastatin group compared with the placebo group, but the differences were not statistically significant; however, the qualitative assessment of seroprotection with a threshold of HBsAb > 10 mIU/mL revealed a significantly higher rate in the case group compared with the control group (80.0% vs. 33.3%; P = 0.025; RR = 2.4, 95% confidence interval (CI): 1.12 - 5.13). On the other hand, the differences in the mean concentrations of the four cytokines, the mean Ct values of the three evaluated genes, and their delta Ct values between the two groups were all insignificant.
| Parameter | Total (N = 30) | Atorvastatin Group (N = 15) | Placebo Group (N = 15) | P-Value |
|---|---|---|---|---|
| HBsAb titer (mIU/mL), Mean (SD) | 296.2 (379.7) | 371.2 (349.5) | 221.2 (405.5) | 0.28 |
| Seroprotection rate, N (%) | 17 (56.7) | 12 (80.0) | 5 (33.3) | 0.02 |
| Cytokine concentration (pg/mL), Mean (SD) | ||||
| TGF-B | 8.7 (8.2) | 9.5 (8.7) | 7.9 (8.0) | 0.60 |
| IL-4 | 0.8 (1.3) | 1.2 (1.7) | 0.3 (0.5) | 0.06 |
| IL-17 | 27.0 (17.0) | 27.8 (18.8) | 26.1 (15.5) | 0.79 |
| IFN- γ | 48.0 (35.0) | 51.3 (34.3) | 44.7 (37.2) | 0.61 |
| Ct value, Mean (SD) | ||||
| GATA3 | 33.4 (2.7) | 33.6 (2.5) | 33.1 (3.0) | 0.59 |
| T-Bet | 34.1 (3.9) | 33.5 (4.3) | 34.6 (3.5) | 0.45 |
| RORc | 26.3 (1.8) | 26.0 (2.1) | 26.8 (1.3) | 0.18 |
| Actin | 34.2 (3.3) | 33.8 (3.1) | 34.7 (3.6) | 0.50 |
| Delta Ct value, Mean (SD) | ||||
| GATA3 | - 0.9 (3.4) | - 0.2 (2.8) | - 1.6 (4.0) | 0.28 |
| T-Bet | - 0.2 (4.2) | - 0.3 (4.6) | - 0.1 (3.8) | 0.87 |
| RORc | - 7.9 (3.3) | - 7.9 (3.3) | - 7.9 (3.4) | 0.99 |
Seroprotection Rate of Anti-HBs, Concentration of Measured Cytokines, and Cycle Threshold Values for GATA3, T-Bet, RORc, and Actin at Follow-up
To make a qualitative comparison between the two groups, considering the Ct values of evaluated genes, Ct values less than 29 were considered as abundant presence of the target nucleic acid, Ct values 30 - 37 were considered as moderate presence of the target nucleic acid, and Ct values greater than 38 were considered as minimal presence. The results of this comparison are presented in Table 3, which shows that the qualitative comparisons also yielded no significant difference between the two groups.
| Parameter | Atorvastatin Group (N = 15) | Placebo Group (N = 15) | P-Value |
|---|---|---|---|
| GATA3 Ct value, N (%) | 0.21 | ||
| ≤ 29 (Abundant) | 0 (0.0) | 1 (6.7) | |
| 30 - 37 (Moderate) | 13 (86.7) | 14 (93.3) | |
| ≥ 38 (Minimal) | 2 (13.3) | 0 (0.0) | |
| T-Bet Ct value, N (%) | 0.54 | ||
| ≤ 29 (Abundant) | 2 (13.3) | 1 (6.7) | |
| 30 - 37 (Moderate) | 13 (86.7) | 14 (93.3) | |
| ≥ 38 (Minimal) | 0 (0.0) | 0 (0.0) | |
| RORc Ct value, N (%) | >0.99 | ||
| ≤ 29 (Abundant) | 15 (100.0) | 15 (100.0) | |
| 30 - 37 (Moderate) | 0 (0.0) | 0 (0.0) | |
| ≥ 38 (Minimal) | 0 (0.0) | 0 (0.0) | |
| Actin Ct value, N (%) | 0.88 | ||
| ≤ 29 (Abundant) | 1 (6.7) | 1 (6.7) | |
| 30 - 37 (Moderate) | 12 (80.0) | 11 (73.3) | |
| ≥ 38 (Minimal) | 2 (13.3) | 3 (20.0) |
Qualitative Comparison of Cycle Threshold Values for GATA3, T-Bet, RORc, and Actin at Follow-up Between the Atorvastatin and Placebo Groups
| Parameter | Total (N = 30) | Immune (N = 17) | Not Immune (N = 13) | P Value |
|---|---|---|---|---|
| Age (y), Mean (SD) | 29.7 (9.4) | 32.2 (11.4) | 26.5 (4.5) | 0.10 |
| BMI (kg/m2), Mean (SD) | 25.2 (4.0) | 24.6 (3.7) | 26.1 (4.3) | 0.33 |
| Male gender, N (%) | 25 (83.3) | 14 (82.4) | 11 (84.6) | 0.87 |
| Smoker, N (%) | 5 (16.7%) | 2 (11.8%) | 3 (23.1) | 0.62 |
| Cytokine Concentration (pg/mL), Mean (SD) | ||||
| TGF-B | 8.7 (8.2) | 7.3 (5.8) | 10.5 (10.7) | 0.29 |
| IL-4 | 0.8 (1.3) | 1.1 (1.6) | 0.4 (0.7) | 0.19 |
| IL-17 | 26.9 (17.0) | 25.7 (15.9) | 28.4 (18.8) | 0.68 |
| IFN- γ | 48.0 (35.0) | 52.3 (36.5) | 42.2 (34.2) | 0.44 |
| Ct Value, Mean (SD) | ||||
| GATA3 | 33.4 (2.7) | 33.3 (2.9) | 33.4 (2.5) | 0.87 |
| T-Bet | 34.1 (3.9) | 33.8 (4.0) | 34.4 (3.9) | 0.68 |
| RORc | 26.3 (1.8) | 26.5 (1.8) | 26.1 (1.8) | 0.51 |
| Actin | 34.3 (3.3) | 34.2 (3.8) | 34.4 (2.7) | 0.86 |
| Delta Ct Value, Mean (SD) | ||||
| GATA3 | - 0.9 (3.4) | - 0.9 (3.5) | - 0.9 (3.5) | 0.97 |
| T-Bet | - 0.2 (4.2) | - 0.3 (4.8) | 0.04 (3.4) | 0.80 |
| RORc | - 7.6 (3.2) | - 8.2 (3.8) | - 7.9 (2.4) | 0.60 |
Univariate Analyses for Relationships Between Immunity and General Characteristics of the Subjects Along With the Concentration of Measured Cytokines, and Cycle Threshold Values for Assessed Genes
Univariate analyses were performed to evaluate the relationship between induced immunity in the subjects with their general characteristics, the concentration of assessed cytokines and expression of target genes. There were no significant relationships between any of these evaluated variables and the level of produced antibodies to induce immunity. Hence, the only factor that was associated with protective antibody levels was short term atorvastatin treatment with a relative risk of 2.4 (95% CI: 1.12 - 5.13). Since univariate analysis did not reveal any possible confounding factor, there was no need to perform further multivariate analysis on the data.
4. Discussion
The effects of short-term atorvastatin treatment on efficacy of HB vaccination in non-responder subjects were assessed in the current study, and the possible molecular pathways in this process were investigated. The main indication for administration of statins was high serum cholesterol levels and the drug reduced cardiovascular events and mortality in this setting (15, 16). Recent studies show that statins can also interfere with Th1/Th2 balance as well as MHC class II expression, which can lead to changes in immune responsiveness (11, 17-19). In this regard, many studies evaluated the effects of this drug on various immunologic-related conditions such as rheumatologic diseases (20-23), response to different vaccines (12, 14, 24), central nervous system autoimmune diseases (25-27), and chronic obstructive pulmonary disease (28).
Based on the promising results reported by the available literature, the current study aimed at assessing the effects of atorvastatin on immune response to HB vaccine in subjects with insufficient antibody titers. Although there were no studies exactly similar to the current study to compare the results with, the current study findings were quite compatible with a previous study focusing on tetanus vaccinations (12). In their randomized, placebo-controlled trial, Lee et al., included 20 healthy subjects and assigned them to a 10-day treatment with either atorvastatin or placebo with a tetanus toxoid (TT) booster on the 5th day. They observed a three-fold higher production of anti-TT antibodies in the atorvastatin group compared with the placebo group, 15 days after vaccination. Further assessments showed that the antibodies were predominantly IgG1, while the level of other IgG subclasses did not go through a significant change. Post-vaccination rise in lymphocyte and platelet counts was also suppressed by the drug (12). Although in the current study the increase in antibody titers was not significantly different between the two groups, a significantly higher rate of protective antibody levels were observed in subjects treated with atorvastatin; and based on the calculated relative risk, it could be stated that the chance of obtaining a protective antibody level was 2.4 times higher in participants taking atorvastatin than the subjects treated with placebo.
On the contrary, in another study conducted by Packard et al., the effects of short-term atorvastatin treatment were evaluated on 150 healthy subjects receiving one dose of hepatitis A vaccine and immune response was assessed one-month post-vaccination. These researchers did not find any significant difference in the level of antibodies between the case and control groups and reported no positive or negative effects exerted from atorvastatin treatment (14). Major aspects of this study differed from those of the current study that might have led to the different outcomes: First, Packard et al., included healthy subjects that had not received hepatitis A vaccine before participation in the study, while the current study included subjects who had not responded to the routine three dose HB vaccination and were considered as non-responders. Second, they started statin treatment on the same day of vaccination; hence, the drug may not have had sufficient time to exert its immunomodulatory actions during antibody production processes.
Overall, multiple immune cell classes cooperate to induce an immune response against a foreign antigen. This virus can be an intra- or extra-cellular pathogen or similar to that of the current study can be injected into the body through vaccination. When activated by the cytokines, B-lymphocytes transform into memory B-cells or plasma cells. Most antigens activate these B-lymphocytes through stimulation of various Th cells (29). Th1 cells are one of these cells in which T-bet gene encodes the T-box 21 master transcription factor that controls production of IFN-γ pro-inflammatory cytokine and plays an important role in differentiation of T-lymphocytes (30, 31). The released IFN-γ activates macrophages and production of opsonization antibodies in B-lymphocytes. These cells typically induce a cell-mediated immune response against intra-cellular pathogens (29, 32).
In another pathway, GATA3 gene encodes a T-cell specific transcription factor known as GATA binding protein 3, which stimulates Th2 cells to release IL-4, IL-5, and IL-13 and also inhibits differentiation of T-cells into Th1 cells (33). The release of these cytokines stimulates B-lymphocytes to produce neutralizing antibodies. These cells induce an immune response mostly against extra-cellular organisms and mediate eosinophil inflammation and allergic responses.
TGF-β and IL-6, secreted from T regulatory cells, stimulate T-cells to differentiate into Th17 cells responsible for producing IL-17. This cytokine is a pro-inflammatory factor and plays an important role in inter-cellular signaling systems (34). Inside these cells, RAR-related orphan receptor (RORc) encodes two isoforms of RORγt and RORγ, transcription factors that attach to the DNA and stimulate differentiation of thymocytes into Th17 cells (35). Th17 cells activate mostly neutrophils and to some extent the B-lymphocytes.
Considering these pathways and to determine how the balance of Th cells shift in atorvastatin-induced immune response after vaccination, the current study measured the concentration of IL-4, IL-17, IFN-γ, and TGF-β cytokines and expression of their corresponding T-Bet, RORc and GATA3 genes in healthy non-responders to HB vaccine. But, the results showed no significant differences in these parameters between the two study groups. These findings were compatible with those of the study conducted by Lee et al., in which the researchers evaluated the effects of atorvastatin on the acute phase response after vaccination by measuring the levels of ESR, CRP, α1-antitrypsin, α1-acid glycoprotein along with Th1 (IFN-γ) and Th2 (IL-4, IL-6, IL-10) cytokines and found no significant differences between the two groups regarding the serum concentration of these cytokines and acute phase parameters (12).
The current study further assessed all the possible factors that might affect reaching a protective HBsAb level in the subject and found that only the study group had a significant correlation with a sufficient immune response in the subjects, which confirmed the significant effect of atorvastatin treatment in reaching protective antibody levels. On the other hand, the findings of the current study showed that none of the assessed immune pathways were responsible for this effect. Therefore, further investigations are required to find the molecular mechanisms through which atorvastatin induces immune response in subjects not responding to HB vaccination. The current study had multiple limitations that should be addressed. First and most importantly, limited financial resources precluded the study from measuring the levels of evaluated cytokines and expression of their corresponding genes at baseline, before atorvastatin treatment. Such measures could provide higher valued information regarding the changes of these parameters after intervention. The current study could also measure the levels of serum cholesterols to have an objective mean to show that the patients took their tablets as instructed. Moreover, following up the participants for a longer period of time and measuring HBsAb levels in different time points could show the trend of changes and the long-term effects of atorvastatin on producing protective antibody levels. Accordingly, it is recommended that these limitations be considered in future studies on this subject.
4.1. Conclusion
It seems that short-term atorvastatin treatment can lead to changes in immune response towards reaching protective antibody levels against HB after vaccination in non-responders, but the immune response induced by this drug was not stimulated through the pathways including IL-4, IL-17, IFN-γ, and TGF-β cytokines and expression of their corresponding T-Bet, RORc, and GATA3 genes.