1. Background
According to the world health organization (WHO), approximately 240 million people worldwide are affected by chronic hepatitis (1, 2). Each year, more than 686,000 patients obtain HBV-related cirrhosis and die of liver cancer (1). In addition, HBV challenges the National health systems and imposes a significant economic burden. Some of the direct costs include the delivery of diagnostic services, hospitalization, and the administration of drugs, whilst indirect costs are generated by the loss of quality of life and reduced productivity. The HBV prevalence rate significantly varies among different regions: It is less than 1% in America and Western Europe, whilst the highest prevalence rate is 5% to 10% and can be observed in East Asia and sub-Saharan Africa. A rate between 2% and 5% could be found in the Middle East and the Indian subcontinent (2-4).
Hepatitis B Virus can be transmitted both vertically and horizontally through contact with infected blood or body fluids. Risk factors for developing HBV infection include having unprotected sex with multiple sexual partners or with infected individuals, being a man, who has sex with other men, sharing needles during intravenous (IV) drug use, living with infected individuals, being occupationally exposed to blood, and travelling to regions, in which HBV is endemic, among others (5).
Hepatitis B Virus should be seriously taken into consideration during pregnancy, as the infection can have clinical implications for both the mother and the fetus (6, 7): without prophylaxis, the risk of vertical transmission is, indeed, high, ranging from 10% to 40% in HBsAg-positive HBeAg-negative mothers to 70% in HBsAg- and HBeAg-positive mothers (8). An effective vaccine is currently available, yet, unfortunately, immunization coverage is not very high, especially in developing countries (9). In order to investigate the HBV prevalence rate in pregnant females, many studies have been conducted worldwide. However, to the best of our knowledge, a comprehensive review of the HBV prevalence rate in pregnant female taking into account the different geographical areas and their socio-economic status is still lacking. This would be of crucial importance for HBV prevention and control programs. Recently, in May 2016, the world health organization (WHO) adopted the “Global Health Sector Strategy on Viral Hepatitis, 2016 to 2021” and one of the strategies suggested was “formulating evidence-based policy and data for action” (10).
As such, this systematic review and meta-analysis was conducted in order to systematically collect available data focusing on HBV prevalence rate in pregnant females from different parts of the world. The current findings could have important implications for health policy- and decision-makers as well as for the stakeholders to better understand the current status of HBV in pregnant females.
2. Methods
2.1. Information Sources and Search
Different electronic databases including Embase, PubMed/MEDLINE, Scopus and ISI/Web of Science were searched from January 1st 2000 to July 31st 2016, using relevant keywords such as “prevalence” or “seroprevalence” or “epidemiology” and “pregnancy” or “pregnant” or “antenatal” in combination with “hepatitis B virus” or “HBV” with no language restrictions. Four authors independently screened titles and abstracts of studies and checked whether they met the inclusion criteria, selecting potentially eligible studies. Any disagreements between reviewers were resolved after discussion involving a fifth referee. This study also consulted the reference list of each potentially relevant study as well as related studies and extant reviews/overviews in order to increase the chance of obtaining all the pertinent studies.
2.2. Eligibility Criteria
Inclusion criteria, according to the PICO criteria, were as follows: i) population-based study reporting HBV prevalence rate in pregnant females with no age limit; ii) studies using a standardized/validated diagnostic test for the detection of HBV in pregnant females; iii) prevalence rate clearly stated or, if missing, appropriate and relevant quantitative information in order to calculate the prevalence data; iv) full-text available or abstract providing minimum relevant quantitative information; and v) primary research studies published in peer-reviewed journals between January 2000 and July 2016, reporting HBV prevalence rate in pregnant females.
Concerning the exclusion criteria, the research did not select studies with pregnant females from high risk groups, such as drug abusers, Human Immunodeficiency Virus (HIV)-positive pregnant females, females attending sexually-transmitted-disease (STDs) clinics, sex workers and dialysis patients. Further exclusion criteria were: i) studies with unclear prevalence data and/or methodological errors, and ii) non primary research studies.
The methodological quality of the included studies was evaluated using the Newcastle-Ottawa scale (NOS) (11). Two authors independently performed the quality assessment and any disagreements between them were resolved by discussion and/or involving a third person as a judge. Up to four stars were attributed on the basis of selection, up to two stars for study comparability and up to three stars on the basis of the study outcome(s). Studies that received two or three stars and two stars for the selection and comparability items, respectively, were considered as affected by a low or moderate risk of bias, while studies that received a star for the selection, comparability and outcome(s) items or achieved null score in all sections were characterized by a high risk of bias.
From studies meeting the inclusion criteria, the following data were extracted by two independent reviewers: namely, surname of the first author, year of study publication, number of women with positive hepatitis B surface antigen (HBsAg), study type, country, sample size, mean age or age range of participants, and HBV prevalence rate. Any disagreements were resolved by discussion. The main outcome of the present study was the HBV prevalence rate in pregnant females. This study was carried out according to the “Preferred Reporting Items for Systematic reviews and Meta-Analysis” (PRISMA) guidelines (12). The study protocol of this systematic review was deposited in the “International Prospective Register of Systematic Reviews” and registered as CRD42016041985 (13).
On the basis of the data provided by the included studies, the pooled prevalence rate with 95% confidence interval (CI) was calculated using the DerSimonian and Laird random-effect model (14, 15). Heterogeneity was estimated carrying out the I2 test (16). In order to evaluate the a priori effects of the pooled HBV prevalence rate in pregnant females, stratified analyses were performed based on factors such as socio-economic status of the study country (countries classified in developed or developing countries according to the International Monetary Fund or IMF), the year of publication of studies, the quality of studies, the geographic areas as classified by the WHO, and mean age or age range of participants (17).
In order to investigate the causes of the heterogeneity between studies, meta-regression analyses were performed based on the sample size and year of publication. To assess the robustness of results, a sensitivity analysis was carried out (18). Studies were ranked according to year of publication and sample size and cumulative meta-analysis was performed to evaluate the impact of these factors (19). Publication bias was determined both visually, inspecting the eventual asymmetry of the funnel plot, and by carrying out the Egger’s test (20).
The relationship between pooled HBV prevalence rate in pregnant females and the human development index (HDI) for each studied country was estimated with linear meta-regression. The HDI is a composite index that combines different measures and indicators, such as life expectancy, educational index, and Gross National Income per capita (GNP) as computed by the united national development program (UNDP).
For all the statistical analyses, figures with a P value of < 0.05 were considered as statistically significant. All statistical analyses were carried out using the open-source software R (version 3.3.1, the R Foundation for Statistical Computing, https://www.R-project.org/) with the Meta package.
3. Results
3.1. Study Flow-Chart and Characteristics of the Included Studies
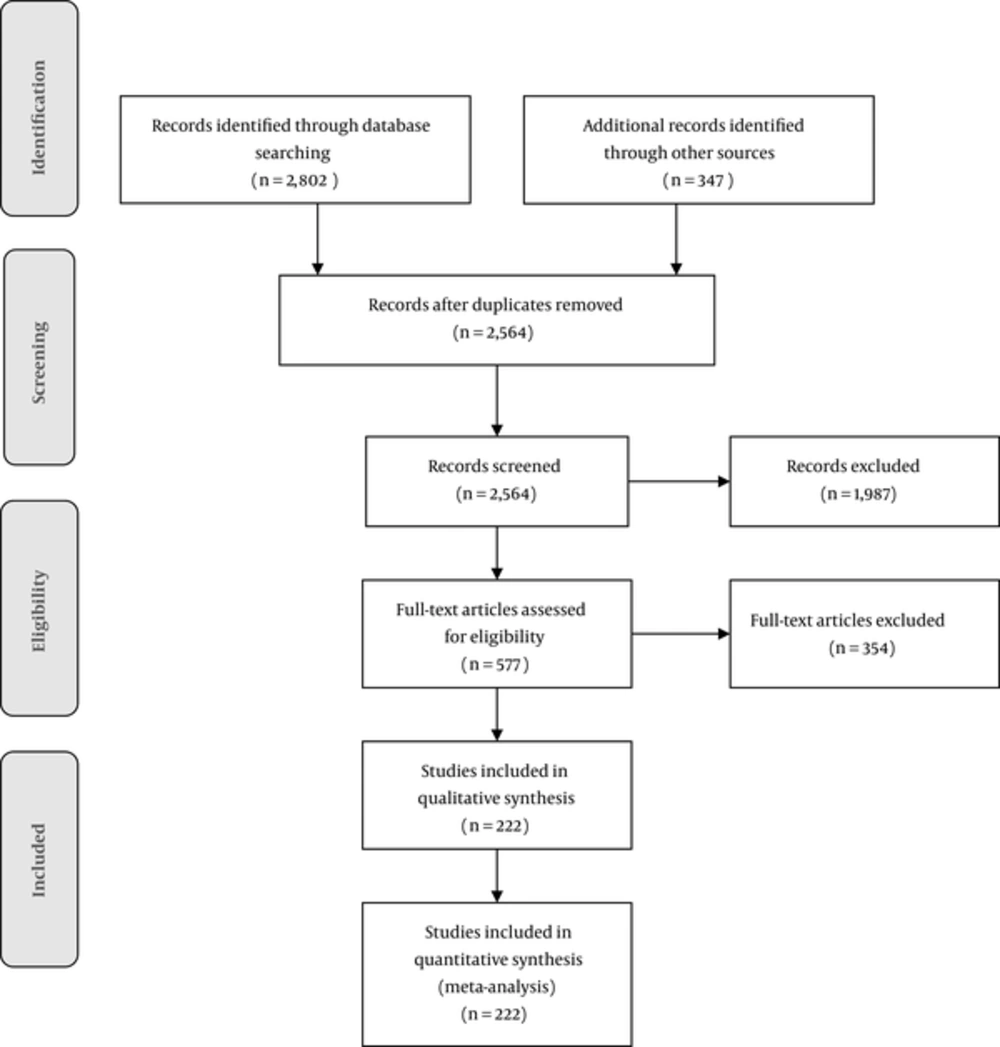
Overall, 3149 studies were identified in the initial search, then after removing duplicate items, 2564 studies were assessed and screened regarding the basis of their title and abstract. Finally, 223 studies met the inclusion criteria, with a total sample size of 40, 958, and 838 pregnant females (Figure 1). The main characteristics of included studies are reported in supplementary file Appendix 1, to which the reader is referred for further details.
According to the WHO geographical regions, 79, 46, 30, 28, 23, and 16 studies were carried out in African, European, Eastern Mediterranean, South East Asiatic, American, and Western Pacific areas, respectively.
3.2. Risk of Bias of Included Studies
The quality assessment using the NOS instrument showed that 152 and 71 studies were characterized by a low and high risk of bias, respectively. In particular, 174, 195, and 87 studies were characterized by low quality in terms of selection, comparability and outcomes, respectively. Overall, 13, 15 and 21 presented medium quality concerning selection, comparability and outcomes, respectively, whilst 35, 12 and 14 exhibited high quality, for selection, comparability, and outcomes, respectively.
3.3. Pooled Prevalence Rate of Hepatitis B Virus in Pregnancy
The overall HBV prevalence rate in pregnant females worldwide, estimated using a random-effect model, was 3% [95%CI 2% - 4%]. Heterogeneity between studies was significantly high (I2 = 99.9%, P < 0.0001) due to epidemiological differences among populations in terms of HBV prevalence, risk factors, and health policies.
3.4. Results of Sub-Groups Analysis of Included Studies
Sub-group analyses pooling HBV prevalence rate in pregnant females based on the study country, WHO areas, IMF classification, quality of the published studies, and study design are shown in Table 1.
| Variables | Number of Studies | Number of Participants | Poled Prevalence Rate (95% CI) | I2 | P Value |
|---|---|---|---|---|---|
| Continent | |||||
| Africa | 81 | 79,450 | 6 (5 - 7) | 96.4 | < 0.000 |
| Asia | 78 | 490,372 | 3 (2 - 3) | 99.5 | < 0.000 |
| Europe | 37 | 1,051,387 | 1 (1 - 2) | 99.6 | < 0.000 |
| South America | 19 | 142,731 | 1 (0 - 2) | 99.6 | < 0.000 |
| North America | 4 | 39,177,247 | 0 (0 - 1) | 99.8 | < 0.000 |
| Oceania | 3 | 17,651 | 2 (2 - 2) | 0 | 0.78 |
| Country socio-economic status | |||||
| Developing | 156 | 478,118 | 4 (3 - 4) | 98.5 | < 0.000 |
| Developed | 66 | 40,480,720 | 1 (1 - 2) | 100 | < 0.000 |
| Risk of bias | |||||
| Low risk | 151 | 40,665,166 | 3 (2 - 4) | 99.9 | < 0.000 |
| High risk | 71 | 293,672 | 3 (2 - 4) | 99.2 | < 0.000 |
| Year of publication | |||||
| 2000 - 2005 | 24 | 262,676 | 2 (1 - 3) | 99.7 | < 0.000 |
| 2006 - 2010 | 54 | 309,111 | 3 (2 - 3) | 99.5 | < 0.000 |
| 2011 - 2016 | 144 | 40,387,051 | 3 (2 - 4) | 99.9 | < 0.000 |
| WHO regional classification | |||||
| AFRO | 79 | 76,950 | 6 (5 - 7) | 96.3 | < 0.000 |
| SEARO | 28 | 196,904 | 2 (1 - 3) | 99.1 | < 0.000 |
| EURO | 46 | 1,104,262 | 1 (1 - 2) | 99.5 | < 0.000 |
| EMRO | 30 | 130,190 | 2 (2 - 3) | 95.4 | < 0.000 |
| PAHO | 23 | 39,319,978 | 1 (0 - 1) | 99.9 | < 0.000 |
| WPRO | 16 | 130,554 | 6 (5 - 8) | 99.2 | < 0.000 |
| Type of study | |||||
| Case – control | 5 | 1,995 | 5 ( 3 - 9) | 80.8 | 0.0003 |
| Cohort | 2 | 6,295 | 7 ( 7 - 8) | 0 | 0.3642 |
| Cross – sectional | 188 | 1,508,658 | 3 (3 - 4) | 99.5 | 0.0001 |
| Prospective | 7 | 18,795 | 3 (2 - 6) | 96.3 | 0.0001 |
| Retrospective | 20 | 39,423,095 | 1 (0 - 3) | 100 | 0.0001 |
Results of Sub-Group Analyses
3.5. Results of Sensitivity and Cumulative Analyses
To ensure the robustness of the results, before and after sensitivity analyses were carried out, showing a statistically unchanged prevalence of 3% [95%CI 2 to 4%]. Also, cumulative meta-analyses, based on the ranking of the sample-size and the year of publication of studies, were conducted. The results did not change statistically.
3.6. Meta-Regression Analyses
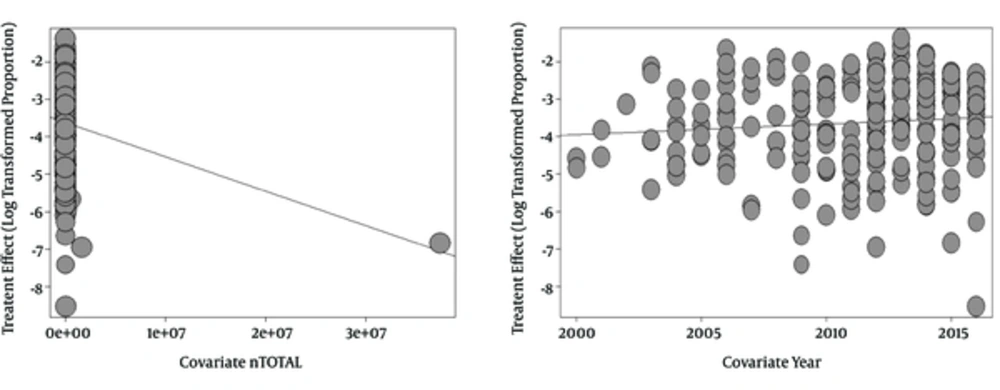
The findings of the meta-regression analyses based on year of publication and sample-size of the included studies are shown in Table 2 and Figure 2.
| Variable | Estimated | Standard Error | Z Value | P Value | Lower CI95% | Upper CI95% |
|---|---|---|---|---|---|---|
| Year | 0.0277 | 0.0332 | 0.8341 | 0.4042 | -0.0374 | 0.0928 |
| Sample-size | -0.000 | 0.000 | -2.3775 | 0.0174 | -0.0000 | -0.0000 |
Results of Meta-Regression Analyses
3.7. Publication Bias
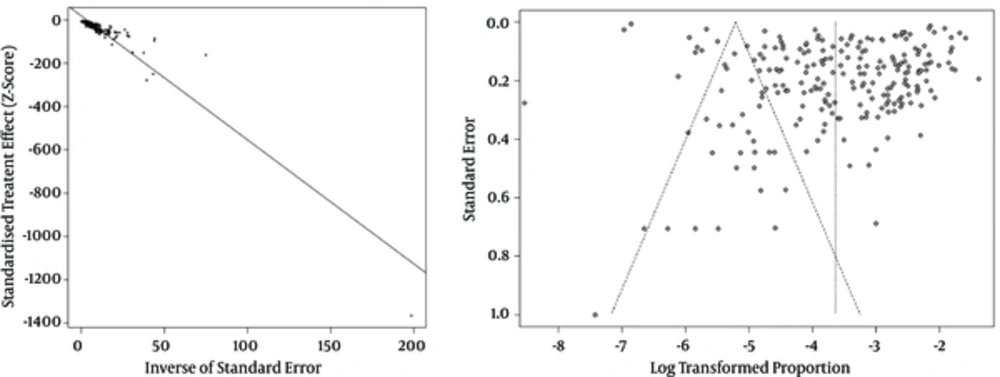
Publication bias analysis showed evidence of publication bias (Egger’s test P = 0.000), as shown in Figure 3.
3.8. Relationship between Hepatitis B Virus prevalence and Human Development Index
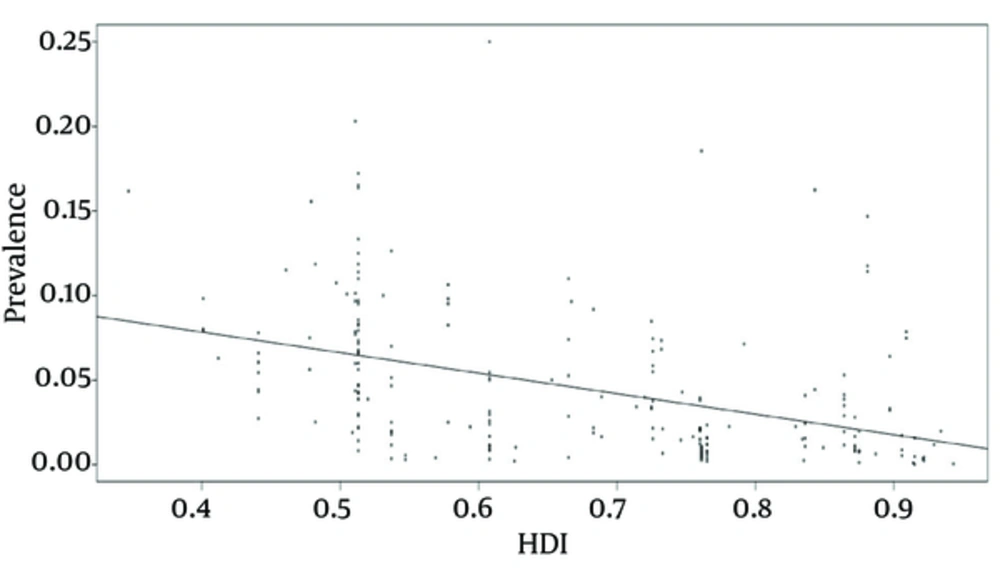
This research found a statistically significant relationship between the pooled HBV prevalence in pregnant females and the HDI of each study country (R = -0.12132, P < 0.000) (Figure 4).
4. Discussion
Despite recent scientific advancements and clinical progress in anti-viral therapy, HBV still represents a major issue worldwide, especially in developing countries (21, 22). The current study has found a pooled HBV prevalence rate of 3% [95%CI 2% - 4%] among pregnant females worldwide.
Generally speaking, HBV vaccination represents an effective tool for reducing HBV-related clinical and epidemiological burden. Specifically, focusing on HBV in pregnancy, this infection is particularly unique and challenging. The key strategies for preventing transmission from pregnant females to their fetuses are by early birth dose and infant vaccination, as well as by the use of Hepatitis B Immunoglobulin (HBIG) and the screening and diagnosis of mothers at high risk and the subsequent use of anti-viral agents (such as nucleoside/nucleotide analogues) during pregnancy in order to reduce maternal DNA concentrations to undetectable concentrations (23). Hepatitis B Virus vaccines in pregnancy have been proven safe, with no side-effects in newborns, since no congenital defects or abnormal developmental patterns have been observed. On one hand, HBV immunization during pregnancy is technically and logistically challenging in that the classical schedule (at 0, 1, and 6 months) is difficult to complete given the limited gestational time. On the other hand, accelerated schedules (at 0, 1, and 4 months) have shown an adequate immunological and protective profile. Intra-dermal route is another promising approach that could be used in high-risk patients, such as subjects with celiac disease (24) as well as in non-responder individuals in order to accelerate the formation of an immunological response and exhibit a protective and adequate safety profile even in pregnancy (25). However, different surveys have underlined low maternal vaccine uptake and have found that many misconceptions and prejudices about HBV in pregnancy do exist. Educational interventions have focused on benefits to both maternal and neonatal health, which should be made in order to increase maternal knowledge related to HBV for improving vaccine confidence and, subsequently, vaccine coverage (7).
Furthermore, infant vaccination is particularly recommended in countries characterized by high HBV endemicity. Concerning anti-virals, according to a recently published meta-analysis, pharmacological treatment is able to effectively reduce peri-natal or mother-to-child transmission, as defined by infant HBSAg seropositivity (risk ratio or RR = 0.3 [95%CI 0.2 - 0.4]) or infant HBV DNA seropositivity (RR = 0.3 [95%CI 0.2 - 0.5]) at 6 to 12 months, with no side-effects (in terms of congenital malformation rate, prematurity rate, and Apgar score) (26).
Combined, multifaceted strategies integrating screening, prevention, and treatment have been proven to be effective in reducing and mitigating HBV-related burden (27-30). As such, health authorities should effectively implement programs and approaches to increase maternal health literacy and to better control HBV in pregnancy. Furthermore, researchers should design high-quality investigations, covering this topic, which is often underestimated and overlooked in the extant literature.
Decision- and policy-makers should take into account the context and the setting for better design and implementation of ad hoc interventions and strategies. The findings of this study showed, indeed, a significant relationship between HDI and prevalence of HBV among pregnant females (P < 0.05). Furthermore, HDI, as introduced by the UND, is a standardized summary measure of social and economic development of countries (31). In many studies, HDI has been found to be a robust, reliable predictor of maternal and infant mortality rate, worldwide (32). In different studies, included in the present systematic review and meta-analysis, maternal high educational levels correlated with a higher awareness of baby's health and resulted in healthier behaviors during pregnancy and delivery, such as attending primary care services and performing HBV screening (33). Low socio-economic status was significantly associated with higher HBV prevalence rate and lower screening adhesion among pregnant females (34, 35).
Concerning the current study, this systematic review and meta-analysis, despite having different strengths like the pre-designed protocol, comprehensive and broad database search, use of different independent reviewers and data extractors, and a rigorous methodological approach, had some drawbacks and limitations that should be acknowledged. These include the high heterogeneity between studies and the fact that approximately a third of the included studies presented high risk of bias.
4.1. Conclusions
Our systematic review and meta-analysis has scrutinized all the scholarly literature from 2000 to 2016 and has captured all the relevant studies reporting HBV prevalence in pregnant females. This study has practical implications for policy- and decision-makers, warranting the adoption and implementation of ad hoc programs and strategies based on epidemiological data and on scientific evidences.