1. Background
Drug-induced hepatitis is a prevalent and serious adverse reaction characterized by liver damage resulting from substances and metabolites such as herbal medicines, biologics, chemical drugs, and environmental toxins. It is a leading cause of acute liver failure (1). Elderly individuals, often burdened with comorbidities like cardiovascular and cerebrovascular diseases, frequently necessitate long-term or combination drug therapies, rendering them more vulnerable to drug-induced hepatitis (2). Surveys indicate that the incidence of drug-induced liver injury in China is 23.8/100,000, with a rate of 20% among the elderly (3). Therefore, studying the characteristics of drug-induced liver injury is crucial for identifying hepatotoxic drugs, discontinuing their usage promptly, and averting adverse reactions.
2. Objectives
Against this backdrop, this study retrospectively analyzed clinical data from elderly patients with drug-induced hepatitis, scrutinized clinical variances among different drug groups, and examined related factors impacting prognosis, with the aim of providing a foundation for clinical diagnosis and treatment.
3. Methods
This study received approval from the institutional review board of Shaoxing People’s Hospital and adhered to the ethical standards outlined in the declaration of Helsinki. Before data acquisition, all participants provided informed written consent.
3.1. Study Subjects
A retrospective analysis was conducted on the clinical data of 140 elderly patients with drug-induced hepatitis admitted to our hospital from June 2021 to June 2023. The inclusion criteria were as follows: (1) meeting the diagnostic criteria for the disease (4); (2) age ≥ 60 years; (3) no interruption in drug treatment before the onset of liver injury; (4) normal liver function before drug treatment; (5) absence of extra-pulmonary tuberculosis; (6) complete clinical data; (7) no use of immunosuppressive agents. Exclusion criteria included: (1) mental abnormalities; (2) autoimmune liver diseases, viral hepatitis, alcoholic liver disease, etc.; (3) other acute infections; (4) liver cirrhosis or fatty liver, etc. Among the 140 patients, there were 78 males and 62 females, aged 60 to 85 years, with an average age of 66.19 ± 2.81 years. The time from drug use to onset was 9.35 ± 1.44 days.
3.2. Research Methods
General information of the patients was collected, including age, gender, types of drugs causing drug-induced hepatitis, underlying diseases, disease severity, main clinical manifestations, clinical typing, treatment, and outcomes.
3.3. Clinical Typing
According to international consensus (5), drug-induced liver injury can be categorized into three types: (1) cholestatic type: Serum alkaline phosphatase (ALP) is normal, and the ratio of alanine aminotransferase (ALT)/ALP is less than 2; (2) hepatocellular type: Alanine aminotransferase exceeds twice the upper limit of normal, ALP is normal, or ALT/ALP > 5; (3) mixed type: Both ALT and ALP are elevated, with ALT more than twice that of normal levels, and the ALT/ALP ratio is between 2 and 5.
3.4. Severity of Drug-Induced Hepatitis
(1) grade 0: No liver damage occurs, there is a certain tolerance to the drug used, and no hepatotoxic reaction is observed (6); (2) grade 1: Serum ALT and/or ALP are increasing, with total bilirubin (TBIL) values within 2.5 - 42.75 μmol/L; symptoms such as fatigue, nausea, anorexia, rash, and pruritus are generally present; (3) grade 2: Serum ALT and/or ALP are elevated, TBIL ≥ 2.5 × ULN, or if TBIL is not elevated, INR ≥ 1.5; symptoms are pronounced; (4) grade 3: Alanine aminotransferase and/or ALP are elevated, TBIL reaches or exceeds 5 times the ULN (i.e., 5 mg/dL or 85.5 μmol/L), with or without INR ≥ 1.5; at this point, the patient's symptoms worsen, requiring hospitalization or prolonging hospital stay; (5) grade 4: Alanine aminotransferase and/or ALP levels are elevated, TBIL reaches or exceeds 10 times the upper limit of normal (i.e., 10 mg/dL or 171 μmol/L), or daily increase ≥ 1.0 mg/dL (17.1 μmol/L); if INR ≥ 2.0 or prothrombin activity (PTA) < 40%, other organ dysfunction related to drug-induced liver injury may occur, such as ascites; (6) grade 5: Results in death or requires liver transplantation to maintain life.
3.5. Treatment
Among the 140 patients in this study, after discontinuing suspected drugs, treatment primarily focused on lowering enzymes, protecting the liver, and relieving jaundice. Drugs such as reduced glutathione, magnesium isoglycyrrhizinate, bicyclol, adenosylmethionine disulfate tosilate, polyene phosphatidylcholine, and deoxynucleotides were commonly used for treatment. Some patients received glucocorticoid therapy. For patients with cholestatic and mixed types, ursodeoxycholic acid treatment was also added.
3.6. Treatment Efficacy
(1) markedly effective: Clinical symptoms were relieved, and abnormal liver function indicators returned to normal (5); (2) effective: Symptoms were alleviated, and abnormal liver function indicators did not completely return to normal, but at least three indicators decreased by more than 60%, and TBIL did not further increase; (3) ineffective: No significant improvement in clinical symptoms, liver function indicators did not reach the level of markedly effective or effective indicators, or showed repeated fluctuations; (4) death. Markedly effective and effective were classified as effective treatment, while ineffective and death were classified as ineffective treatment.
3.7. Observation Indicators
Analyze the overall clinical characteristics and types of drugs causing drug-induced hepatitis. Examine the differences in clinical symptoms, typing, severity, and liver function indicators [total bilirubin (TBIL), aspartate aminotransferase (AST), ALT, ALP] caused by different drugs. Additionally, analyze the related factors affecting prognosis.
3.8. Statistical Analysis
The statistical package for social science (SPSS) 24.0 software (IBM, Armonk, NY, USA) was used for data analysis. Measurement data were expressed as (x̅ ± s). One-way analysis of variance was used for multiple group comparisons, and t-tests were used for pairwise comparisons between multiple groups. Count data were expressed as cases (n), and chi-square tests were used. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Clinical Characteristics of 140 Patients with Drug-Induced Hepatitis
Among the 140 patients, various gastrointestinal symptoms were observed, including loss of appetite in 35 cases (25.00%), nausea and vomiting in 39 cases (27.86%), poor appetite in 43 cases (30.71%), and fatigue in 37 cases (26.43%). Jaundice was present in 90 cases (64.29%), itching in 29 cases (20.71%), rash in 18 cases (12.86%), and fever in 41 cases (29.29%). Elevated levels of TBIL were found in 65 cases (43.92%), ALT in 138 cases (98.57%), and ALP in 96 cases (68.57%).
4.2. Common Types of Drugs Causing Drug-Induced Liver Injury and Clinical Subtype Characteristics
Among the 140 cases, the common types of drugs causing drug-induced liver injury included Chinese herbal medicine in 48 cases (34.29%), antituberculosis drugs in 39 cases (27.86%), and antibiotics in 33 cases (23.57%). Clinical subtypes were hepatocellular type in 102 cases (72.86%), mixed liver injury type in 17 cases (12.14%), and cholestatic type in 21 cases (15.00%). Please refer to Table 1 for details.
| Drug Type | Cases | Specific Drugs | Clinical Typing | ||
|---|---|---|---|---|---|
| Hepatocellular Type | Mixed Liver Injury | Cholestatic Type | |||
| Anti-tuberculosis drugs | 39 (27.86) | Isoniazid, rifampicin, ethambutol, pyrazinamide, etc. | 32 (82.05) | 6 (15.38) | 1 (2.57) |
| Chinese herbal medicine | 48 (34.29) | Polygonum multiflorum, tripterygium wilfordii, huoxiang zhengqi liquid, cold medicine, medicated wine, etc. | 41 (85.42) | 4 (8.33) | 3 (6.25) |
| Antibiotics | 33 (23.57) | Levofloxacin, piperacillin-tazobactam sodium, clarithromycin, amoxicillin, cefoperazone-sulbactam, cefclox, etc. | 20 (60.61) | 2 (6.06) | 11 (33.33) |
| Cardiovascular drugs | 8 (5.71) | Aspirin, statins, propafenone, clopidogrel, etc. | 4 (50.00) | 2 (25.00) | 2 (25.00) |
| Analgesics | 4 (2.86) | Paracetamol, betamethasone, ibuprofen, etc. | 3 (75.00) | 0 (0.00) | 1 (25.00) |
| Antitumor drugs | 2 (1.43) | Cisplatin, paclitaxel, etc. | 1 (50.00) | 0 (0.00) | 1 (50.00) |
| Immunosuppressants | 1 (0.71) | Infliximab | 0 (0.00) | 1 (100.00) | 0 (0.00) |
| Digestive system drugs | 2 (1.43) | Omeprazole, rabeprazole | 1 (50.00) | 1 (50.00) | 0 (0.00) |
| Neurological and psychiatric drugs | 1 (0.71) | Flupenthixol melitracen tablets | 0 (0.00) | 1 (100.00) | 0 (0.00) |
| Antithyroid drugs | 1 (0.71 | Propylthiouracil | 0 (0.00) | 0 (0.00) | 1 (100.00) |
| Others | 1 (0.71) | Health products | 0 (0.00) | 0 (0.00) | 1 (100.00) |
| Total | 140 | - | 102 (72.86 %) | 17 (12.14) | 21 (15.00) |
a Values are expressed as No (%).
4.3. Comparison of Clinical Symptoms, Severity, and Liver Function Indicators in Patients with Different Types of Drug-Induced Hepatitis
There were no significant differences in the occurrence of clinical symptoms such as loss of appetite, fatigue, nausea/vomiting, pruritus, rash, and fever among the three groups (P > 0.05). This indicates that the prevalence of these symptoms did not differ significantly between patients exposed to different types of drugs. However, the incidence of jaundice in the anti-tuberculosis drug group was significantly higher than in the traditional Chinese medicine and antibiotic groups (P < 0.05, Table 2). This suggests that patients exposed to anti-tuberculosis drugs were more likely to develop jaundice compared to those exposed to other types of drugs.
| Groups | Cases | Appetite Loss | Fatigue | Nausea/Vomiting | Poor Appetite | Jaundice | Itching | Rash | Fever |
|---|---|---|---|---|---|---|---|---|---|
| Anti-TB drugs | 39 | 14 (35.90) | 12 (30.77) | 13 (33.33) | 17 (43.59) | 31 (79.49) | 7 (17.95) | 6 (15.38) | 12 (30.77) |
| Chinese herbal medicine | 48 | 10 (20.83) | 14 (29.17) | 10 (20.83) | 11 (22.92) | 24 (50.00) b | 16 (33.33) | 8 (16.67) | 13 (27.08) |
| Antibiotics | 33 | 8 (24.24) | 9 (27.27) | 11 (33.33) | 12 (36.36) | 17 (51.52) b | 8 (24.24) | 2 (6.06) | 12 (36.36) |
| χ2 | - | 2.634 | 0.106 | 2.216 | 4.326 | 9.161 | 2.718 | 2.114 | 0.790 |
| P | - | 0.268 | 0.948 | 0.330 | 0.115 | 0.010 | 0.257 | 0.347 | 0.674 |
a Values are expressed as No (%).
b P < 0.05 compared to anti-TB drugs.
Furthermore, the analysis revealed significant differences in the severity of drug-induced hepatitis across the three groups. The incidence of grade 1 drug-induced hepatitis was significantly lower with traditional Chinese medicine compared to anti-tuberculosis drugs and antibiotics (P < 0.05), while the incidence of grade 3 drug-induced hepatitis was significantly higher with traditional Chinese medicine compared to anti-tuberculosis drugs and antibiotics (P < 0.05, Table 3). This indicates that the severity of drug-induced hepatitis varied depending on the type of drug exposure.
a Values are expressed as No (%).
b P < 0.05 compared to Chinese Herbal Medicine.
Lastly, there were no significant differences in liver function indicators among the three groups (P > 0.05, Table 4). This suggests that the levels of liver function indicators such as TBIL, ALT, and ALP did not vary significantly between patients exposed to different types of drugs.
| Group | Cases | TBIL (μmol/L) | ALT (U/L) | ALP (U/L) | AST (U/L) |
|---|---|---|---|---|---|
| Anti-TB drugs | 39 | 183.47 ± 32.47 | 194.39 ± 68.79 | 588.47 ± 189.36 | 124.78 ± 97.46 |
| Chinese herbal medicine | 48 | 178.45 ± 34.58 | 197.69 ± 43.45 | 561.69 ± 144.58 | 140.69 ± 97.58 |
| Antibiotics | 33 | 169.79 ± 37.65 | 196.78 ± 59.69 | 572.69 ± 127.46 | 134.27 ± 87.43 |
| F | - | 1.400 | 0.038 | 0.316 | 0.303 |
| P | - | 0.251 | 0.963 | 0.730 | 0.739 |
a Values are expressed as mean ± SD.
4.4. Comparison of Clinical Indicators of Drug-Induced Hepatitis in Patients with Different Treatment Efficacies
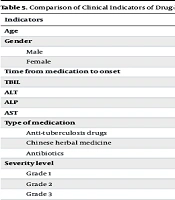
Out of 140 patients, 127 (90.71%) showed effective treatment after symptomatic treatment, while 13 (9.29%) showed ineffective treatment. There were significant differences in TBIL, ALT, ALP, AST, the use of anti-tuberculosis drugs, and severity between patients with effective and ineffective treatment (P < 0.05, Table 5).
| Indicators | Effective Treatment (N = 127) | Ineffective Treatment (N = 13) | χ2/t | P |
|---|---|---|---|---|
| Age | 66.16 ± 2.90 | 66.54 ± 1.85 | 0.462 | 0.645 |
| Gender | ||||
| Male | 71 | 7 | 0.020 | 0.887 |
| Female | 56 | 6 | ||
| Time from medication to onset | 9.31 ± 1.47 | 9.69 ± 1.18 | 0.902 | 0.369 |
| TBIL | 86.69 ± 17.65 | 189.78 ± 34.78 | 17.935 | < 0.001 |
| ALT | 82.61 ± 21.42 | 193.47 ± 24.86 | 17.511 | < 0.001 |
| ALP | 262.47 ± 63.78 | 547.78 ± 121.18 | 13.868 | < 0.001 |
| AST | 61.78 ± 24.73 | 121.78 ± 78.69 | 6.221 | < 0.001 |
| Type of medication | ||||
| Anti-tuberculosis drugs | 32 (25.20) | 7 (53.85) | 4.816 | 0.028 |
| Chinese herbal medicine | 45 (35.43) | 3 (23.08) | 0.799 | 0.371 |
| Antibiotics | 30 (23.62) | 3 (23.08) | 0.002 | 0.965 |
| Severity level | 56.248 | < 0.001 | ||
| Grade 1 | 29 (22.83) | 0 (0.00) | ||
| Grade 2 | 49 (38.58) | 1 (7.69) | ||
| Grade 3 | 47 (37.01) | 5 (38.46) | ||
| Grade 4 | 2 (1.57) | 7 (53.85) | ||
| Grade 5 | 0 (0.00) | 0 (0.00) | ||
| Clinical subtype | 5.339 | 0.086 | ||
| Hepatocellular | 89 (70.08) | 13 (100.00) | ||
| Cholestatic | 17 (13.39) | 0 (0.00) | ||
| Mixed Injury | 21 (16.54) | 0 (0.00) | ||
| Number of hepatoprotective drugs | 1.317 | 0.518 | ||
| 1 type | 47 (37.01) | 3 (23.08) | ||
| 2 type | 41 (32.28) | 6 (46.15) | ||
| 3 type | 39 (30.71) | 4 (30.77) |
a Values are expressed as No. (%) or mean ± SD.
4.5. Analysis of Factors Influencing the Prognosis of Drug-induced Hepatitis Patients
Logistic analysis (with the dependent variable coded as 0 for effective treatment and 1 for ineffective treatment) showed that post-treatment TBIL, ALT, ALP, AST, and severity were associated with the prognosis of drug-induced hepatitis patients (P < 0.05, Table 6).
| Factor | β | SE | Wald χ2 | p | OR | 95 %CI |
|---|---|---|---|---|---|---|
| Post-treatment TBIL | 0.171 | 0.042 | 16.577 | < 0.001 | 1.186 | 1.093 ~ 1.288 |
| Post-treatment ALT | 0.168 | 0.057 | 8.687 | 0.003 | 1.183 | 1.058 ~ 1.323 |
| Post-treatment ALP | 0.154 | 0.053 | 8.443 | 0.004 | 1.166 | 1.051 ~ 1.294 |
| Post-treatment AST | 0.147 | 0.054 | 7.410 | 0.007 | 1.158 | 1.042 ~ 1.288 |
| Use of anti-tuberculosis drugs | 1.124 | 1.456 | 2.596 | 0.441 | 0.177 | 0.177 ~ 53.397 |
| Severity | 2.470 | 0.524 | 22.219 | < 0.001 | 11.822 | 4.233 ~ 33.018 |
5. Discussion
The pathogenesis of drug-induced hepatitis mainly involves two aspects: Drug toxicity and allergic reactions. On one hand, drugs or their metabolites can generate potentially toxic compounds catalyzed by P450 enzymes (7). If these toxic substances bind to liver cells, they can cause liver damage. Drugs or their metabolites can also bind to specific proteins in the liver in the form of semi-antigens, forming complete antigens. This process leads to the generation of antibodies in the body, triggering allergic reactions, and ultimately causing liver damage (8, 9).
In the elderly, due to decreased absorption, metabolism, and distribution capacity of drugs, drug-induced liver injury is more likely to occur. For example, reduced gastric acid secretion may affect the dissolution and decomposition of some drugs in the stomach, slowing their absorption and delaying the time to peak blood concentration. Additionally, elderly individuals have a decreased proportion of body water and increased fat content. Consequently, serum protein levels, particularly albumin levels, decrease with age, making elderly populations more susceptible to toxic reactions with prolonged or high-dose protein application. Furthermore, elderly patients experience reduced renal blood flow, decreased glomerular filtration rate, and diminished renal tubular reabsorption function, with reduced glomerular filtration being the main cause of impaired drug metabolism (10, 11). Drug-induced hepatitis can be caused by various medications, including Chinese herbal medicine, antineoplastic drugs, and antipyretic analgesics, with different types prevalent in different countries and regions. In Western countries, antibiotics, cardiovascular drugs, and antituberculosis drugs are the top three medications causing drug-induced hepatitis. In contrast, in Eastern countries, the common culprits are Chinese herbal medicine, antituberculosis drugs, and antibiotics (12). Antituberculosis therapy often requires prolonged treatment, and the combined use of multiple drugs may increase hepatotoxicity and lead to severe hepatitis (13). As a major user of traditional Chinese medicine (TCM) globally, China generally perceives TCM as harmless and natural, which has led to the widespread misuse of Chinese herbal decoctions, contributing to liver injury events (14). Common Chinese herbal medicines causing drug-induced liver injury in elderly patients include Paris polyphylla, Herba Euphorbiae, Polygonum multiflorum, and compound preparations for treating conditions such as osteoarthritis, rheumatoid arthritis, diabetes, rheumatism, and psoriasis (15-17). These medicines are often decoctions and patent medicines made from various herbs, making it challenging to accurately identify the specific Chinese medicines used (18). Additionally, it is difficult to pinpoint which specific herbs or chemical components cause the liver injury.
The results of this study show that among the 140 cases, the most common drugs were Chinese herbal medicine (48 cases, 34.29%), antituberculosis drugs (39 cases, 27.86%), and antibiotics (33 cases, 23.57%). Chinese herbal medicine predominated in this study, possibly due to the tendency of the Chinese population to use traditional Chinese medicine for self-regulating health. The clinical classification was mainly of the hepatocellular type, and there were no significant differences in the occurrence of clinical symptoms such as loss of appetite, fatigue, nausea, vomiting, itching, rash, and fever, or liver function indicators among patients of different drug types (19). Logistic regression analysis showed that TBIL, ALT, ALP, AST, and severity were associated with the prognosis of drug-induced hepatitis patients. Considering that the hepatotoxicity and pathogenesis of most Chinese and Western medicines are still unclear, close observation of patients' clinical manifestations and liver function indicators during drug treatment is necessary (20). Once liver function damage is detected, drug therapy should be discontinued immediately, and active treatment should be initiated. Further research is needed to investigate the relationship between different types of drugs and the pathological characteristics of liver injury.
While this study provides valuable insights into the clinical characteristics of drug-induced hepatitis in elderly patients and the causative drugs, it is important to acknowledge some limitations and areas for further research. Firstly, the retrospective design employed in this study may have led to data incompleteness and information bias, potentially impacting the stability and representativeness of the results. Secondly, despite including a certain number of cases, the sample size remains limited, which may restrict the ability to detect rare drugs or clinical features. Additionally, the data originated from the medical records of a single healthcare institution, lacking support from multicenter or multi-region data, which may reduce the generalizability of the findings. Moreover, due to the lack of adequate control over potential confounding factors such as underlying diseases and medication history, caution should be exercised in interpreting and generalizing the results. Furthermore, this study did not delve into the molecular mechanisms of drug-induced liver injury, which warrants further elucidation through future laboratory research. Lastly, geographical limitations and the absence of a control group also constrain the interpretation and generalization of the results.
Therefore, future studies should consider improving study design, increasing sample size, fostering multicenter collaboration, and exploring mechanisms, among other aspects, to address these limitations and achieve a more comprehensive understanding of the pathogenesis and clinical characteristics of drug-induced hepatitis in elderly patients.
In summary, Chinese herbal medicine, antituberculosis drugs, and antibiotics are the most common drugs causing drug-induced hepatitis in elderly patients, with the hepatocellular type being the most prevalent clinical subtype. The prognosis of patients is associated with liver function, drug type, and severity. During treatment, careful selection of appropriate drugs and determination of appropriate drug dosages based on the patient's condition are crucial. Furthermore, it is essential to enhance health education for the elderly population, improve their understanding of drugs (including health supplements and herbal medicines) to minimize misuse and overuse, and pay attention to observing and managing adverse reactions, thereby reducing the occurrence of drug-induced hepatitis. Further research is needed to investigate the relationship between different types of drugs and the pathological characteristics of liver injury.