1. Background
Although significant progress has been made in the prevention and treatment of chronic Hepatitis B (CHB), Hepatitis B virus (HBV) infection remains a major global public health problem. In 2019, there were approximately 296 million people with chronic HBV infection worldwide, and about 820,000 people die annually from HBV infection-related diseases (1). Inflammatory and fibrotic lesions in liver tissue promote disease progression in HBV-infected patients and guide clinical decisions regarding the timing of antiviral therapy.
The long-term persistence of chronic inflammation related to HBV infection causes hepatic tissue damage and limits self-repair, leading to the development of hepatic fibrosis (2). Moreover, long-term chronic inflammation is significantly associated with the progression of HBV to cirrhosis and hepatocellular carcinoma (3). Hepatic fibrosis can be reversed in the early stages with timely and effective treatment. Therefore, early identification and diagnosis of hepatic histopathological changes, coupled with timely antiviral therapy, can slow or even reverse disease progression.
The current indication for antiviral therapy in HBV patients is mainly based on alanine aminotransferase (ALT) levels, HBV deoxyribonucleic acid (DNA) levels, and liver disease severity (4). However, studies have shown that ALT levels are not directly related to liver tissue inflammatory activity, indicating that patients with normal ALT levels may still have liver histological changes and even require antiviral therapy (5, 6). Liver biopsy is usually used in such cases to determine the extent of liver disease. However, liver biopsy is an invasive examination, a high-risk, complex, and unrepeatable method with high sampling error, making it difficult to become a routine screening tool.
In recent years, several researchers have used imaging and serum testing indicators to construct non-invasive diagnostic models for histopathological evaluation (7-9). These models provide an important basis for clinical diagnosis and treatment, and the accuracy of diagnosing hepatic fibrosis has been greatly improved through the combined application of multiple indicators and models. However, the degree of liver tissue inflammation is not directly related to fibrosis in CHB patients. Studies have shown that liver inflammation is an independent risk factor affecting the accuracy of fibrosis staging diagnostics (10). Fibrosis diagnostic models are poorly suited to assessing the degree of hepatic tissue inflammatory activity. Additionally, very few non-invasive models can be used to diagnose the degree of Hepatitis in CHB patients.
2. Objectives
This study retrospectively analyzed the relevant CHB cases reported by the Third People's Hospital of Shenzhen from 2016 to 2019 to provide a reference basis for the diagnosis and treatment of related diseases.
3. Methods
3.1. Ethical Approval
The patients signed an informed consent to receive liver tissue puncture examination to determine the degree of hepatic histopathological changes, and the study was approved by the Ethics Committee of The Third People's Hospital of Shenzhen (No. 2018-038).
3.2. Patient Selection
A total of 91 CHB patients treated at the Third People's Hospital of Shenzhen from January 2016 to December 2019 were retrospectively selected. The inclusion criteria were: Patients who met the diagnostic criteria of the Guidelines for the Prevention and Treatment of CHB (2015 Updated Edition), with a previous history of CHB or Hepatitis B surface antigen (HBsAg) positivity for > 6 months and who had not received antiviral treatment. Exclusion criteria were: Patients who had other viral Hepatitis (Hepatitis A, C, D, and E), alcoholic/non-alcoholic Hepatitis, drug-induced Hepatitis, autoimmune liver disease, human immunodeficiency virus infection, malignant tumors, decompensated cirrhosis, other major organ damage diseases; patients who had received anti-HBV treatment or immunomodulators; psychiatric patients; pregnant and breastfeeding patients; patients participating in other interventional studies.
3.3. Diagnostic Criteria and Grouping
The diagnosis followed the pathological diagnostic criteria for chronic Hepatitis, which were revised based on the Scheuer scoring system for inflammation grading (G0-G4) and fibrosis staging (S0-S4) in the results of liver puncture (11). G0 indicates no inflammation in and around the confluent area and in the lobules; G1 indicates inflammation in the confluent area, degeneration, and a few foci of necrosis in the lobules; G2 indicates mild fragmentary necrosis in and around the confluent area, degeneration, pitting, focal necrosis, or eosinophilic vesicles in the lobules; G3 indicates moderate fragmentary necrosis in and around the confluent area, degeneration, and necrosis in the lobules or seen in the bridging necrosis; and G4 indicates severe fragmentary necrosis in and around the confluent area, extensive bridging necrosis in the lobules, involvement of multiple lobules, and structural derangement of the lobules. S0 indicates no fibrosis; S1 indicates enlargement of the confluent area and fibrosis; S2 indicates fibrous septum formation with preservation of lobular structure; S3 indicates fibrous septum with disorganization of the lobular structure and no cirrhosis; and S4 indicates early cirrhosis or definite cirrhosis. The patients were classified into mild (G1-2, S0-2, 39 cases), moderate (G3, S1-3, 30 cases), and severe (G4, S2-4, 22 cases) chronic Hepatitis groups based on the G and S outcomes. Notably, the higher value was selected as the total inflammatory activity when G and S were inconsistent.
3.4. Research Methods
The clinical data of the enrolled patients, including basic information (gender, age, height, and weight), blood routine tests (white blood cell (WBC), and platelet (PLT)), liver function tests (ALT, aspartate aminotransferase (AST), glutamyl transpeptidase (GGT), albumin (ALB), alkaline phosphatase (ALP), glucose (GLU), total bilirubin (TB), triglyceride (TG), and uric acid (UA)), liver fibrosis tests (hyaluronidase (HA), procollagen III (PIIIP), type IV collagen (CIV), and laminin (LN)), inflammatory markers (C-reactive protein (CRP)), tumor marker (alpha-fetoprotein (AFP)), HBV markers (HBV DNA, HBsAg, and Hepatitis B envelope antigen (HBeAg)), and imaging findings (portal vein internal diameter (PVID) and spleen thickness (ST)) were collected from the hospital’s electronic medical record system. APRI (ALT-to-PLT ratio index) score was calculated as follows: (AST/ULN)/PLT (109/L) × 100 (ULN represents the upper normal limit of AST). AAR (AST-to-ALT ratio) was calculated as follows: AST/ALT. GPR (GGT-to-PLT ratio) score was calculated as follows: (GGT/ULN)/PLT (109/L) × 100 (ULN represents the upper normal limit of GGT). FIB-4 (Fibrosis index based on the four factors) score was calculated as follows: (age × AST)/(PLT × ALT0.5), S-index was calculated as follows: 1000 × GGT/(PLT × ALB2).
3.5. Statistical Analysis
SPSS 26.0 statistical software was used for data analysis. Quantitative data with normal distribution were expressed as means ± standard deviations. Comparisons among multiple groups were first performed using one-way ANOVA. Two-way comparisons were then performed using the LSD-t-test if the difference between the groups was statistically significant. Quantitative data with non-normal distribution were represented as the central tendency by M (P25 ~ P75). Kruskal-Wallis H-test and Dunn-t-test were used for comparison among multiple groups. Qualitative data were expressed as relative numbers, and comparisons between groups were made using the χ2 test. Spearman rank correlation analysis was used to assess the correlation between two variables. Factors with P < 0.15 in the univariate analysis were included in the logistic regression analysis using the forward-biased likelihood ratio stepwise regression method for the construction of the risk prediction models. The differential diagnostic performance of the relevant indicators was evaluated using the receiver operator characteristic (ROC) curve. The accuracy of the relevant indicators was assessed based on sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The performance of different ROC curves was compared using the DeLong test. P < 0.05 was considered a statistically significant difference.
4. Results
4.1. Non-invasive Diagnostic Indicators in CHB Patients with Different Liver Histopathological Degrees
4.1.1. Baseline Data Analysis
A total of 79 of the 91 patients were male, of which 38, 26, 21, and 6 patients had liver tissue inflammation graded as G1, G2, G3, and G4, respectively. Moreover, 13, 27, 29, and 22 cases were fibrosis staged as S1, S2, S3, and S4, respectively. About 61.54% (16/26), 30.77% (8/26), 85.19% (23/27), and 62.96% (17/27) of patients with normal ALT levels had G ≥ 2, G ≥ 3, S ≥ 2, and S ≥ 3, respectively. Comparative analysis at the first examination on admission (Table 1) showed that the average age of the patients increased with the degree of Hepatitis. Moreover, WBC, PLT, UA, HA, LN, PVID, and ST were significantly different among patients with different Hepatitis groups (P < 0.05). WBC and PLT levels were significantly lower in the severe Hepatitis group than in the mild group, while HA, LN, PVID, and ST levels were significantly higher in the severe group than in the mild group. The levels of other indicators were not statistically significant at baseline among the three groups (P > 0.05).
| Descriptive Item | Mild Group (n = 39) | Moderate Group (n = 30) | Severe Group (n = 22) | t/H Value | P-Value |
|---|---|---|---|---|---|
| Age, y | 36.41 ± 8.02 | 38.30 ± 7.14 | 40.00 ± 9.27 | 1.448 | 0.241 |
| Male | 34 (87.2) | 25 (83.3) | 20 (90.9) | 0.644 | 0.725 |
| BMI (kg/m2) | 24.17 ± 2.28 | 24.67 ± 4.00 | 22.75 ± 3.36 | 2.141 | 0.125 |
| WBC (109/L) | 6.44 (5.83 ~ 7.93) a | 5.98 (4.78 ~ 6.73) | 5.16 (4.32 ~ 6.49) b | 9.137 | 0.010 |
| PLT (109/L) | 187.0 (142.0 ~ 233.0) a | 176.0 (133.0 ~ 190.5) | 121.0 (71.8 ~ 175.8) b | 12.173 | 0.002 |
| ALT (U/L) | 62.7 (32.0 ~ 100.0) | 47.5 (38.8 ~ 85.3) | 53.0 (34.0 ~ 58.0) | 1.711 | 0.425 |
| AST (U/L) | 38.0 (27.0 ~ 56.0) | 37.0 (27.0 ~ 53.5) | 37.0 (31.5 ~ 60.5) | 0.240 | 0.887 |
| GGT (U/L) | 35.0 (24.0 ~ 56.0) | 35.5 (26.5 ~ 62.8) | 51.0 (28.0 ~ 82.0) | 1.855 | 0.396 |
| ALB (g/L) | 44.8 (43.1 ~ 47.2) | 43.9 (42.0 ~ 45.8) | 43.3 (41.9 ~ 46.9) | 2.113 | 0.348 |
| ALP (U/L) | 81.5 (65.8 ~ 105.8) | 78.0 (60.0 ~ 117.0) | 83.5 (61.0 ~ 91.3) | 0.534 | 0.766 |
| GLU (mmol/L) | 5.10 (4.80 ~ 5.45) | 4.95 (4.61 ~ 5.21) | 4.95 (4.59 ~ 5.44) | 2.106 | 0.349 |
| TB (µmol/L) | 14.51 (11.63 ~ 17.90) | 14.55 (10.11 ~ 17.37) | 17.80 (11.65 ~ 22.33) | 4.143 | 0.126 |
| TG (mmol/L) | 1.05 (0.85 ~ 1.68) | 0.95 (0.69 ~ 1.65) | 1.03 (0.71 ~ 1.57) | 1.482 | 0.477 |
| UA (mmol/L) | 362.0 (314.5 ~ 423.5) | 385.3 (345.0 ~ 469.0) a | 327.0 (254.0 ~ 387.0) c | 6.118 | 0.047 |
| CRP (mg/L) | 3.36 (2.85 ~ 5.97) | 2.67 (1.56 ~ 3.92) | 3.28 (1.93 ~ 4.99) | 1.278 | 0.528 |
| AFP (ng/mL) | 2.85 (2.22 ~ 4.75) | 3.80 (2.88 ~ 5.31) | 4.22 (2.40 ~ 5.55) | 2.802 | 0.246 |
| HA (ng/mL) | 78.43 (45.35 ~ 123.60) a | 69.41 (51.22 ~ 128.34) a | 143.70 (76.17 ~ 431.68) b, c | 11.539 | 0.003 |
| PIIIP (ng/mL) | 21.75 (18.02 ~ 25.95) | 23.91 (19.44 ~ 27.78) | 26.42 (18.72 ~ 61.12) | 3.750 | 0.153 |
| CIV (ng/mL) | 20.75 (18.33 ~ 26.03) | 23.62 (17.69 ~ 27.62) | 27.80 (19.70 ~ 56.36) | 4.405 | 0.111 |
| LN (ng/mL) | 28.73 (25.86 ~ 38.37) a | 34.47 (31.19 ~ 44.56) | 48.56 (30.82 ~ 72.66) b | 10.281 | 0.006 |
| HBV DNA (log10, IU/mL) | 5.87 (4.55 ~ 7.43) | 5.95 (3.77 ~ 7.44) | 5.49 (3.18 ~ 6.81) | 1.351 | 0.509 |
| HBsAg (IU/mL) | 3649.5 (1558.8 ~ 6774.7) | 4273.0 (1822.0 ~ 8502.6) | 1936.0 (1384.2 ~ 4962.4) | 2.524 | 0.283 |
| HBeAg (S/CO) | 7.11 (0.41 ~ 902.08) | 3.56 (0.55 ~ 685.98) | 9.72 (0.44 ~ 99.30) | 0.362 | 0.835 |
| PVID (mm) | 11.0 (10.0 ~ 12.0) a | 11.0 (11.0 ~ 12.3) | 11.9 (10.8 ~ 13.0) b | 7.424 | 0.024 |
| ST (mm) | 33.0 (30.0 ~ 36.3) a | 34.8 (31.7 ~ 42.1) | 40.3 (35.8 ~ 46.1) b | 10.491 | 0.005 |
Abbreviations: CHB, chronic Hepatitis B; BMI, Body Mass Index; WBC, white blood cell; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, glutamyl transpeptidase; ALB, albumin; ALP, alkaline phosphatase; GLU, glucose; TB, total bilirubin; TG, triglyceride; UA, uric acid; CRP, C-reactive protein; AFP, alpha fetoprotein; HA, hyaluronidase; PIIIP, procollagen III; CIV, type IV collagen; LN, laminin; HBV DNA, Hepatitis B virus deoxyribonucleic acid; HBsAg, Hepatitis B surface antigen; HBeAg, Hepatitis B envelope antigen; PVID, portal vein internal diameter; ST, spleen thickness.
a P < 0.05, compared with severe group.
b P < 0.05, compared with mild group.
c P < 0.05, compared with moderate group.
4.1.2. Correlation Analysis
Spearman’s rank correlation analysis (Table 2) showed that inflammation grading was significantly positively correlated with PVID, ST, TB, LN, AAR, GPR, FIB-4, and S-index, and significantly negatively correlated with Body Mass Index (BMI) and PLT. Furthermore, fibrosis staging was significantly positively correlated with PVID, ST, AFP, HA, PIIIP, CIV, LN, APRI, AAR, GPR, FIB-4, and S-index, while significantly negatively correlated with WBC and PLT. Hepatitis degree grading was significantly positively correlated with PVID, ST, HA, CIV, LN, APRI, GPR, FIB-4, and S-Index, while significantly negatively correlated with WBC and PLT. The correlation coefficients were statistically significant (P < 0.05).
| Descriptive Item | PVID | ST | PLT | WBC | BMI | TB | AFP | HA | PIIIP | CIV | LN | APRI | AAR | GPR | FIB-4 | S-Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation grade | ||||||||||||||||
| Correlation coefficient | 0.272 | 0.288 | -0.267 | NA | -0.226 | 0.217 | NA | NA | NA | NA | 0.258 | 0.189 | 0.179 | 0.252 | 0.228 | 0.232 |
| P-value | 0.009 | 0.007 | 0.011 | NA | 0.045 | 0.043 | NA | NA | NA | NA | 0.028 | 0.073 | 0.090 | 0.017 | 0.030 | 0.028 |
| Fibrosis stage | ||||||||||||||||
| Correlation coefficient | 0.283 | 0.273 | -0.251 | -0.302 | NA | NA | 0.249 | 0.265 | 0.282 | 0.235 | 0.342 | 0.210 | 0.234 | 0.230 | 0.322 | 0.224 |
| P-value | 0.007 | 0.010 | 0.016 | 0.004 | NA | NA | 0.030 | 0.023 | 0.016 | 0.045 | 0.003 | 0.045 | 0.026 | 0.029 | 0.002 | 0.035 |
| Hepatitis degree | ||||||||||||||||
| Correlation coefficient | 0.289 | 0.334 | -0.349 | -0.316 | NA | NA | NA | 0.317 | NA | 0.235 | 0.378 | 0.234 | 0.201 | 0.224 | 0.308 | 0.217 |
| P-value | 0.006 | 0.001 | 0.001 | 0.003 | NA | NA | NA | 0.006 | NA | 0.045 | 0.001 | 0.026 | 0.056 | 0.034 | 0.003 | 0.041 |
Abbreviations: CHB, chronic Hepatitis B; PVID, portal vein internal diameter; ST, spleen thickness; PLT, platelet; WBC, white blood cell; BMI, Body Mass Index; TB, total bilirubin; AFP, alpha fetoprotein; HA, hyaluronidase; PIIIP, procollagen III; CIV, type IV collagen; LN, laminin; APRI, aspartate aminotransferase-to-platelet ratio index; AAR, aspartate aminotransferase-to-alanine aminotransferase ratio; GPR, glutamyl transpeptidase-to- platelet ratio; FIB-4, fibrosis index based on the four factors; NA, not available.
4.1.3. Evaluation of Diagnostic Performance
The diagnostic performance of the above indicators with significant correlation was evaluated using ROC for inflammation grading, fibrosis staging, and Hepatitis degree grading (Table 3).
| Descriptive Item | AUC (95% CI) | P-Value a | Cut-Off | Sensitivity | Specificity | PPV | NPV | Z-Value | P-Value b |
|---|---|---|---|---|---|---|---|---|---|
| Inflammation grade (G ≥ 3) | |||||||||
| PVID | 0.670 (0.543 ~ 0.796) | 0.011 | 11.05 | 70.4 | 61.9 | 44.19 | 82.98 | 1.887 | 0.059 |
| ST | 0.684 (0.547 ~ 0.821) | 0.007 | 37.05 | 68.0 | 73.0 | 50.00 | 85.19 | 1.667 | 0.096 |
| BMI | 0.640 (0.503 ~ 0.777) | 0.046 | 24.00 | 57.4 | 72.0 | 43.90 | 81.58 | 2.380 | 0.017 |
| PLT | 0.668 (0.544 ~ 0.793) | 0.011 | 129.5 | 84.4 | 48.1 | 56.52 | 79.41 | 1.659 | 0.097 |
| TB | 0.639 (0.496 ~ 0.781) | 0.043 | 19.20 | 48.0 | 88.9 | 63.16 | 81.16 | 2.236 | 0.025 |
| LN | 0.660 (0.520 ~ 0.800) | 0.029 | 67.41 | 30.4 | 98.0 | 88.00 | 75.00 | 1.733 | 0.083 |
| PSBPTL | 0.806 (0.676 ~ 0.936) | 0.000 | 0.347 | 81.8 | 82.9 | 82.00 | 75.00 | ||
| APRI | 0.619 (0.494 ~ 0.745) | 0.073 | 0.525 | 74.1 | 51.6 | 41.00 | 83.00 | 2.786 | 0.005 |
| AAR | 0.613 (0.487 ~ 0.739) | 0.090 | 0.565 | 85.2 | 35.8 | 36.00 | 85.00 | 3.284 | 0.001 |
| GPR | 0.660 (0.544 ~ 0.777) | 0.018 | 0.675 | 65.4 | 67.2 | 45.00 | 83.00 | 1.728 | 0.084 |
| FIB-4 | 0.644 (0.519 ~ 0.768) | 0.031 | 0.935 | 77.8 | 50.0 | 50.00 | 88.00 | 3.105 | 0.002 |
| S-Index | 0.647 (0.531 ~ 0.764) | 0.029 | 0.165 | 57.7 | 71.4 | 45.00 | 80.00 | 1.832 | 0.067 |
| Fibrosis stage (S ≥ 3) | |||||||||
| PVID | 0.663 (0.548 ~ 0.778) | 0.008 | 10.25 | 88.0 | 42.5 | 65.67 | 73.91 | 2.717 | 0.007 |
| ST | 0.658 (0.543 ~ 0.773) | 0.011 | 36.45 | 58.3 | 77.5 | 75.68 | 60.78 | 1.604 | 0.109 |
| WBC | 0.675 (0.563 ~ 0.787) | 0.005 | 6.025 | 70.0 | 61.2 | 71.43 | 59.57 | 2.283 | 0.022 |
| PLT | 0.646 (0.532 ~ 0.760) | 0.017 | 133.5 | 85.0 | 41.2 | 77.78 | 53.13 | 2.163 | 0.031 |
| AFP | 0.647 (0.518 ~ 0.776) | 0.031 | 2.98 | 71.7 | 60.0 | 72.73 | 56.25 | 2.057 | 0.040 |
| HA | 0.654 (0.529 ~ 0.779) | 0.024 | 125.74 | 45.0 | 81.8 | 75.00 | 55.10 | 2.177 | 0.030 |
| PIIIP | 0.664 (0.540 ~ 0.787) | 0.017 | 26.22 | 50.0 | 81.8 | 76.92 | 57.45 | 2.066 | 0.039 |
| CIV | 0.636 (0.510 ~ 0.763) | 0.046 | 23.43 | 57.5 | 72.7 | 71.88 | 58.54 | 1.956 | 0.050 |
| LN | 0.698 (0.577 ~ 0.820) | 0.004 | 30.46 | 80.0 | 57.6 | 69.57 | 70.37 | 1.550 | 0.121 |
| PSWPAHPCL | 0.843 (0.742 ~ 0.943) | 0.000 | 0.529 | 76.5 | 87.0 | 88.46 | 64.52 | ||
| ARPI | 0.623 (0.506 ~ 0.740) | 0.045 | 0.525 | 66.7 | 57.5 | 66.67 | 57.50 | 2.703 | 0.007 |
| AAR | 0.636 (0.520 ~ 0.752) | 0.026 | 0.790 | 51.0 | 75.0 | 72.22 | 54.55 | 2.712 | 0.007 |
| GPR | 0.633 (0.518 ~ 0.749) | 0.030 | 0.675 | 54.0 | 72.5 | 71.05 | 55.77 | 1.876 | 0.061 |
| FIB-4 | 0.687 (0.578 ~ 0.797) | 0.002 | 0.815 | 84.3 | 45.0 | 66.15 | 69.23 | 2.585 | 0.010 |
| S-Index | 0.630 (0.512 ~ 0.747) | 0.036 | 0.135 | 59.2 | 67.5 | 69.05 | 57.45 | 1.822 | 0.069 |
| Hepatitis degree (moderate-to-severe) | |||||||||
| PVID | 0.651 (0.535 ~ 0.767) | 0.014 | 10.25 | 86.3 | 41.0 | 65.67 | 69.57 | 1.965 | 0.049 |
| ST | 0.659 (0.545 ~ 0.773) | 0.011 | 36.45 | 57.1 | 76.9 | 75.68 | 58.82 | 1.362 | 0.173 |
| WBC | 0.678 (0.566 ~ 0.791) | 0.004 | 6.025 | 71.8 | 62.0 | 73.81 | 59.57 | 1.599 | 0.110 |
| PLT | 0.661 (0.547 ~ 0.774) | 0.009 | 192.5 | 46.2 | 82.7 | 67.19 | 66.67 | 1.472 | 0.141 |
| HA | 0.614 (0.485 ~ 0.744) | 0.095 | 158.7 | 26.8 | 96.9 | 91.67 | 50.82 | 2.348 | 0.019 |
| CIV | 0.608 (0.480 ~ 0.737) | 0.114 | 23.43 | 53.7 | 68.7 | 68.75 | 53.66 | 2.041 | 0.041 |
| LN | 0.705 (0.583 ~ 0.827) | 0.003 | 30.46 | 80.5 | 59.4 | 71.74 | 70.37 | 0.995 | 0.320 |
| PSWPHCL | 0.778 (0.668 ~ 0.887) | 0.000 | 0.587 | 59.5 | 90.6 | 86.96 | 63.04 | ||
| APRI | 0.620 (0.503 ~ 0.738) | 0.050 | 0.525 | 67.3 | 59.0 | 68.63 | 57.50 | 2.755 | 0.006 |
| AAR | 0.617 (0.500 ~ 0.734) | 0.057 | 0.790 | 50.0 | 74.4 | 72.22 | 52.73 | 1.761 | 0.078 |
| GPR | 0.630 (0.514 ~ 0.747) | 0.035 | 0.675 | 52.9 | 71.8 | 71.05 | 53.85 | 1.481 | 0.139 |
| FIB-4 | 0.680 (0.568 ~ 0.791) | 0.003 | 0.815 | 84.6 | 46.2 | 67.69 | 69.23 | 1.973 | 0.048 |
| S-Index | 0.626 (0.508 ~ 0.745) | 0.042 | 0.135 | 58.0 | 66.7 | 69.05 | 55.32 | 1.711 | 0.087 |
Abbreviations: AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; PVID, portal vein internal diameter; ST, spleen thickness; BMI, Body Mass Index; PLT, platelet; TB, total bilirubin; LN, laminin; WBC, white blood cell; AFP, alpha fetoprotein; HA, hyaluronidase; PIIIP, procollagen III; CIV, type IV collagen; APRI, aspartate aminotransferase-to-platelet ratio index; AAR, aspartate aminotransferase-to-alanine aminotransferase ratio; GPR, glutamyl transpeptidase-to- platelet ratio; FIB-4, fibrosis index based on the four factors; PSBPTL, portal vein internal diameter + spleen thickness + Body Mass Index + platelet + total bilirubin + laminin; PSWPAHPCL, portal vein internal diameter + spleen thickness + white blood cell + platelet + alpha fetoprotein + hyaluronidase + procollagen III + type IV collagen + laminin; PSWPHCL, portal vein internal diameter + spleen thickness + white blood cell + platelet + hyaluronidase + type IV collagen + laminin.
a Show the P-value of the ROC curve analysis.
b Show the P-value of the Delong test comparing the ROC curve between multifactor combination indicator and others.
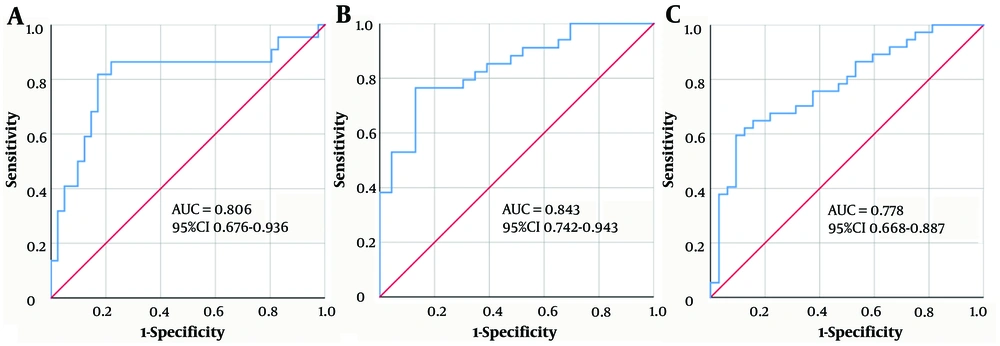
The area under the curve (AUC) of the above indicators for the diagnosis of CHB patients with G ≥ 3, S ≥ 3, or moderate-to-severe Hepatitis ranged from 0.610 to 0.710 with an optimal cut-off value. Diagnostic efficacy analysis (combining the single factors with P < 0.15) further showed that the regression equation established by PSBPTL (PVID + ST + BMI + PLT + TB + LN) to predict the risk of inflammation grade (G ≥ 3) was logit (PPSBPTL) = -3.045 + 0.280 × PVID + 0.030 × ST - 0.135 × BMI - 0.003 × PLT + 0.047 × TB + 0.024 × LN. The regression equation established by PSWPAHPCL (PVID + ST + WBC + PLT + AFP + HA + PIIIP + CIV + LN) to predict the risk of fibrosis stage (S ≥ 3) was logit (PPSWPAHPCL) = -5.778 + 0.092 × PVID + 0.106 × ST - 0.274 × WBC - 0.005 × PLT + 0.229 × AFP - 0.006 × HA + 0.035 × PIIIP + 0.053 × CIV + 0.040 × LN. Finally, the regression equation established by PSWPHCL (PVID + ST + WBC + PLT + HA + CIV + LN) to predict the risk of moderate-to-severe Hepatitis was logit (PPSWPHCL) = -2.593 + 0.326 × PVID + 0.005 × ST - 0.231 × WBC - 0.004 × PLT + 0.002 × HA - 0.015 × CIV + 0.029 × LN. Receiver operator characteristics analysis further showed that the AUC of PSBPTL, PSWPAHPCL, and PSWPHCL were 0.806, 0.843, and 0.778, respectively. These multifactorial combinations of indicators showed better sensitivity, specificity, and performance in terms of PPV than the application of these factors individually or AAR, GPR, FIB-4, and S-index. The DeLong test also showed that their ROC curves performed significantly better than those of APRI, AAR, and FIB-4 (P < 0.05). These results indicate that PSBPTL, PSWPAHPCL, and PSWPHCL have excellent diagnostic accuracy and value (Figure 1).
Receiver operator characteristics (ROC) analysis of multifactorial combinations predicting the risk of G ≥ 3 inflammation (A), S ≥ 3 fibrosis (B), and moderate-to-severe Hepatitis (C) in CHB patients. A, PSBPTL, portal vein internal diameter + spleen thickness + Body Mass Index + platelet + total bilirubin + laminin; B, PSWPAHPCL, portal vein internal diameter + spleen thickness + white blood cell + platelet + alpha fetoprotein + hyaluronidase + procollagen III + type IV collagen + laminin; C, PSWPHCL, portal vein internal diameter + spleen thickness + white blood cell + platelet + hyaluronidase + type IV collagen + laminin.
4.2. The Effectiveness of Antiviral Therapy
4.2.1. Changes in Clinical Biochemical Indicators
After 24 and 72 weeks of treatment, the differences in PLT and HA levels among the groups were statistically significant (P < 0.05). Specifically, PLT levels were lowest in the severe group, while HA levels were highest in the severe group. In addition, HBeAg levels were significantly different among the groups after 72 weeks of treatment. Specifically, HBeAg levels were significantly lower in the severe group than in the mild group (P < 0.05) (Table 4). However, ALT normalization rates were not significantly different among the three groups after 24, 48, 72, and 96 weeks of antiviral treatment (P > 0.05). Nonetheless, ALT normalization rates were higher in the moderate and severe groups than in the mild group at the same time points. Notably, ALT normalization rates of the mild, moderate, and severe groups after 96 weeks of antiviral treatment were 63.2% (12/19), 86.7% (13/15), and 92.3% (12/13), respectively (Table 5).
| Descriptive Item | Mild Group (n = 39) | Moderate Group (n = 30) | Severe Group (n = 22) | H-Value | P-Value |
|---|---|---|---|---|---|
| 24W | |||||
| PLT (109/L) | 182.0 (134.5 ~ 219.8) a | 153.0 (96.5 ~ 169.5) | 97.0 (65.0 ~ 147.0) b | 11.600 | 0.003 |
| ALT (U/L) | 35.0 (22.0 ~ 62.3) | 38.5 (28.0 ~ 47.5) | 26.5 (24.8 ~ 35.0) | 3.896 | 0.143 |
| AST (U/L) | 28.0 (21.5 ~ 37.5) | 33.5 (27.0 ~ 37.0) | 27.5 (23.8 ~ 41.3) | 2.296 | 0.317 |
| GGT (U/L) | 28.0 (19.0 ~ 45.5) | 38.0 (23.0 ~ 90.0) | 36.5 (26.8 ~ 65.3) | 2.406 | 0.300 |
| HA (ng/mL) | 83.92 (82.61 ~ 133.32) | 79.37 (62.85 ~ 88.44) a | 208.95 (124.11 ~ 438.56) c | 12.430 | 0.002 |
| PIIIP (ng/mL) | 20.74 (17.28 ~ 37.82) | 22.28 (16.34 ~ 25.95) | 29.33 (16.69 ~ 55.16) | 1.177 | 0.555 |
| CIV (ng/mL) | 19.24 (18.05 ~ 33.18) | 19.36 (17.97 ~ 25.37) | 28.10 (14.23 ~ 50.16) | 0.736 | 0.692 |
| LN (ng/mL) | 31.92 (31.81 ~ 59.90) | 26.61 (24.04 ~ 33.51) | 45.27 (22.67 ~ 66.02) | 1.966 | 0.374 |
| HBeAg (S/CO) | 2.31 (0.36 ~ 41.19) | 3.21 (1.01 ~ 107.75) | 0.44 (0.37 ~ 1.37) | 3.713 | 0.156 |
| HBV DNA (log10, IU/mL) | 2.00 (2.00 ~ 2.67) | 2.34 (2.00 ~ 3.14) | 2.00 (2.00 ~ 2.18) | 2.561 | 0.278 |
| 72W | |||||
| PLT (109/L) | 210.0 (183.0 ~ 256.0) a, c | 153.0 (122.0 ~ 202.0) b | 114.5 (66.5 ~ 133.0) b | 16.715 | 0.000 |
| ALT (U/L) | 27.5 (18.3 ~ 53.3) | 28.0 (23.0 ~ 34.0) | 27.0 (18.5 ~ 31.0) | 0.983 | 0.612 |
| AST (U/L) | 25.0 (20.3 ~ 34.3) | 25.0 (21.0 ~ 27.0) | 26.0 (20.8 ~ 36.0) | 0.836 | 0.658 |
| GGT (U/L) | 27.0 (20.0 ~ 41.5) | 25.0 (18.0 ~ 32.0) | 28.0 (20.5 ~ 53.5) | 0.192 | 0.908 |
| HA (ng/mL) | 82.46 (53.40 ~ 98.00) | 59.76 (49.83 ~ 77.45) a | 129.02 (72.64 ~ 182.67) c | 8.453 | 0.015 |
| PIIIP (ng/mL) | 22.79 (17.34 ~ 28.54) | 22.25 (15.53 ~ 24.38) | 23.93 (18.57 ~ 45.95) | 2.620 | 0.270 |
| CIV (ng/mL) | 20.02 (19.27 ~ 26.96) | 22.67 (15.59 ~ 25.50) | 23.81 (21.47 ~ 38.10) | 1.324 | 0.516 |
| LN (ng/mL) | 27.35 (17.74 ~ 37.60) | 28.74 (19.48 ~ 39.56) | 32.61 (25.29 ~ 61.05) | 1.575 | 0.455 |
| HBeAg (S/CO) | 4.15 (0.45 ~ 81.52) a | 0.85 (0.33 ~ 4.94) | 0.58 (0.35 ~ 1.27) b | 7.043 | 0.030 |
| HBV DNA (log10, IU/mL) | 2.00 (2.00 ~ 3.08) | 2.00 (2.00 ~ 2.00) | 2.00 (1.69 ~ 2.00) | 3.591 | 0.166 |
Abbreviations: CHB, chronic Hepatitis B; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, glutamyl transpeptidase; HA, hyaluronidase; PIIIP, procollagen III; CIV, type IV collagen; LN, laminin; HBeAg, hepatitis B envelope antigen; HBV DNA, hepatitis B virus deoxyribonucleic acid.
a Compared with severe group, P < 0.05.
b Compared with mild group, P < 0.05.
c Compared with moderate group, P < 0.05.
| Descriptive Item | Hepatitis Degree | χ2-Value | P-Value | ||
|---|---|---|---|---|---|
| Mild (n = 39) | Moderate (n = 30) | Severe (n = 22) | |||
| ALT normalization rates | |||||
| 24 W | 11/20 (55.0) | 9/15 (60.0) | 10/12 (83.3) | 2.748 | 0.253 |
| 48 W | 12/20 (60.0) | 11/17 (64.7) | 10/14 (71.4) | 0.471 | 0.790 |
| 72 W | 14/23 (60.9) | 11/13 (84.6) | 10/13 (76.9) | 2.557 | 0.279 |
| 96 W | 12/19 (63.2) | 13/15 (86.7) | 12/13 (92.3) | 4.746 | 0.093 |
| χ2-value | 0.292 | 4.220 | 2.075 | ||
| P-value | 0.962 | 0.239 | 0.557 | ||
| HBV DNA clearance rate | |||||
| 24 W | 11/16 (68.8) | 7/17 (41.2) | 14/18 (77.8) | 5.370 | 0.068 |
| 48 W | 13/21 (61.9) | 13/19 (68.4) | 14/18 (77.8) | 1.145 | 0.564 |
| 72 W | 17/26 (65.4) | 13/16 (81.3) | 13/16 (81.3) | 1.883 | 0.390 |
| 96 W | 16/22 (72.7) | 24/26 (92.3) | 14/17 (82.4) | 3.258 | 0.196 |
| χ2-value | 0.626 | 14.433 | 0.180 | ||
| P-value | 0.890 | 0.002 | 0.981 | ||
Abbreviations: ALT, alanine aminotransferase; HBV DNA, Hepatitis B virus deoxyribonucleic acid.
4.2.2. Hepatitis B Virus DNA Clearance Rate
Hepatitis B virus DNA levels were significantly lower after 24 weeks of antiviral treatment than before treatment (P < 0.05). Notably, the HBV DNA clearance rate was higher in the mild (68.8%, 11/16) and severe (77.8%, 14/18) groups than in the moderate group (41.2%, 7/17). Furthermore, HBV DNA clearance rates after 48 weeks of antiviral treatment were slightly higher in the moderate and severe groups than in the mild group (P > 0.05). However, HBV DNA clearance rates of patients in the moderate group were significantly different at different time points (P < 0.05), with the highest HBV DNA clearance rate detected after 96 weeks of treatment (92.3%, 24/26), slightly higher compared with the mild and severe groups at the same time points (P > 0.05) (Table 5).
4.2.3. Changes in Virological Indicators
Only two patients in the mild group had achieved HBsAg negative conversion and serological conversion after 96 weeks of antiviral therapy. The HBeAg negative and serological conversion rates were slightly higher in the severe group than in the mild and moderate groups after 24, 48, and 72 weeks of antiviral treatment (P > 0.05). Hepatitis B envelope antigen negative conversion rate of the severe group reached 50.0% (4/8) after 72 weeks of treatment. Hepatitis B envelope antigen serological conversion rate after 96 weeks of antiviral treatment was significantly higher in the severe group than in the other two groups (P < 0.05). Also, only one patient in the mild group achieved HBeAg serological conversion, while about 46.2% (6/13) of patients in the severe group achieved HBeAg serological conversion (Table 6).
| Descriptive Item | Hepatitis Degree | χ2-Value | P-Value | ||
|---|---|---|---|---|---|
| Mild (n = 39) | Moderate (n = 30) | Severe (n = 22) | |||
| HBeAg negative conversion rates | |||||
| 24 W | 2/10 (20.0) | 0/7 (0.0) | 4/8 (50.0) | 5.263 | 0.072 |
| 48 W | 4/19 (21.1) | 1/9 (11.1) | 4/12 (33.3) | 1.500 | 0.472 |
| 72 W | 4/17 (23.5) | 3/11 (27.3) | 4/8 (50.0) | 1.877 | 0.391 |
| 96 W | 7/17 (41.2) | 3/13 (23.1) | 7/13 (53.8) | 2.606 | 0.272 |
| HBeAg serological conversion | |||||
| 24 W | 0/10 (0.0) | 0/7 (0.0) | 1/8 (12.5) | 2.214 | 0.331 |
| 48 W | 0/19 (0.0) | 0/9 (0.0) | 1/12 (8.3) | 2.393 | 0.302 |
| 72 W | 1/17 (5.9) | 0/11 (0.0) | 1/8 (12.5) | 1.386 | 0.500 |
| 96 W | 1/17 (5.9) | 0/13 (0.0) | 6/13 (46.2) | 12.389 | 0.002 |
Abbreviations: HBeAg, Hepatitis B envelope antigen.
5. Discussion
Analysis of pathological changes in hepatic tissue is valuable in assessing disease progression, timely treatment, developing antiviral regimens, and preventing medication irregularities in CHB patients. Presently, the assessment of hepatic inflammatory activity is mainly based on the pathological findings of liver biopsy, which is not suitable for clinical application. Therefore, non-invasive indicators with high diagnostic value are crucial in the clinical diagnosis and treatment of CHB. Although many non-invasive models have been developed for the diagnosis of liver fibrosis, there is no non-invasive diagnostic method that can accurately diagnose the inflammatory activity of liver tissue in HBV patients.
In the present study, the analysis of baseline data showed that the liver tissue of chronic HBV-infected patients with normal ALT levels had developed varying degrees of inflammatory necrosis and fibrosis. Although ALT may directly indicate liver damage, it cannot accurately predict hepatic histopathological alterations (12, 13). Herein, most patients were males, and the severity of Hepatitis was associated with age. Several previous studies have also shown that male gender and age are risk factors for disease progression in patients with HBV infection (14, 15). The results also showed that PLT and HA levels were related to the severity of the disease. The more severe the chronic Hepatitis, the lower the PLT level and the higher the HA level. Correlation analysis showed that inflammation grade, fibrosis stage, or Hepatitis degree was significantly positively correlated with PVID and ST, and significantly negatively correlated with PLT. It is believed that as fibrosis progresses and portal hypertension increases, PLT is trapped and destroyed in the enlarged spleen, resulting in a continuous decrease in PLT levels (16, 17). Several studies have reported that PLT is strongly correlated with the severity of liver injury and may indicate the degree of liver tissue inflammation and fibrosis in HBV-infected patients. Besides, the diagnostic efficiency of PLT in assessing significant liver fibrosis and early cirrhosis is comparable to that of FIB-4 and APRI (18-20). In this study, Hepatitis degree was significantly correlated with HA, CIV, LN, and WBC levels. The best cut-off value for HA was 158.7 ng/mL, with a low sensitivity but a high specificity of 96.9% and PPV of 91.67%. HA is the most important glycosaminoglycan component of the extracellular matrix. Increased blood HA concentration in the early stage of hepatic fibrosis can reflect liver fibrosis and directly reflect the degree of liver function impairment (21). In addition, GPR, FIB-4, and S-index were significantly positively correlated with inflammation grading. Also, GPR, FIB-4, S-index, and APRI were significantly positively correlated with fibrosis staging and Hepatitis degree grading. These results indicate that hematological indicators or ultrasonic measurements can be used to evaluate the liver histopathological degrees, thus guiding clinical intervention. However, the AUC values of these significantly correlated metrics for the diagnosis of hepatic histopathological changes only ranged from 0.610 to 0.710. Multifactorial analysis found that the combination of PSBPTL, PSWPAHPCL, and PSWPHCL had high diagnostic value when used to predict the risk of CHB patients (G ≥ 3, S ≥ 3, moderate-to-severe Hepatitis, respectively), with significantly higher diagnostic accuracy than these factors alone as well as APRI, AAR, GRP, FIB-4, and S-index. However, future studies should include more indicators for the prediction of liver histopathology degree in these patients.
Antiviral therapy is widely used for the treatment of CHB. Antiviral therapy aims to achieve maximum long-term suppression of HBV replication. Durable disappearance of HBsAg after discontinuation of the drug is the desired endpoint of antiviral treatment. Herein, liver function and virological test markers were not significantly different among the three groups at baseline, indicating comparability in the efficacy of antiviral therapy. The findings also showed that HBeAg levels significantly decreased in the severe Hepatitis group after treatment. Previous studies suggested that serum HBeAg levels in patients are indirectly negatively correlated with hepatic inflammation and fibrosis (22, 23). Another study also showed that HBeAg promotes hepatic fibrosis by mediating the inflammatory function of macrophages via toll-like receptor 2 (TLR2) (24). More than half of the patients in the severe Hepatitis group had HBeAg negative conversion and achieved seroconversion after antiviral treatment, possibly due to the differences between the groups. Studies have shown that spontaneous HBeAg seroconversion rates are about 2% - 15% yearly. However, the conversion rates are lower among males, Asians, those under 30 years of age, those who acquired the infection through vertical transmission, patients with normal ALT, and patients without mutations in the core promoter or precursor cells (1, 25). In the present study, ALT normalization rates and HBV DNA clearance rates were higher in the moderate and severe Hepatitis groups than in the mild patients at the corresponding time points, possibly due to the higher HBeAg levels in the mild group. Studies have suggested that elevated serum HBeAg levels may affect the body's immune response to clear HBV. Besides, CHB patients with lower HBeAg levels have higher response rates to antiviral medications and better recovery of liver function indices (26). In this study, only two mild patients achieved HBsAg negative conversion and seroconversion without discontinuation of medication at the last time point. However, future studies should assess whether patients with severe Hepatitis can achieve HBsAg negative conversion with longer antiviral duration. Studies have suggested that old age (over 50 years), male gender, low HBsAg levels, and negative HBeAg are predictive factors for spontaneous clearance of HBsAg (27). In summary, antiviral treatment may have a better effect on patients with severe Hepatitis in the short term.
These results may improve the clinical diagnosis and treatment of CHB patients with different degrees of chronic Hepatitis. However, this study has some limitations. First, this is a retrospective analysis from a single center. Besides, the small sample size, the short time range of enrollment, and the short duration of treatment may lead to some biases. Therefore, future prospective studies should explore this aspect through multicenter and large-sample data to verify the reliability of the model constructed.
In summary, these results suggest that multiple non-invasive indicators are closely associated with the different liver histopathological degrees in patients with chronic HBV infection. PSBPTL, PSWPAHPCL, and PSWPHCL can predict the risk of developing G ≥ 3 inflammation, S ≥ 3 fibrosis, moderate-to-severe Hepatitis, respectively. In addition, short-term antiviral therapy has a more pronounced effect on patients with severe Hepatitis by improving liver inflammation and suppressing viral replication.