1. Background
Hepatitis C virus (HCV) infection is an ongoing health problem affecting a large number of individuals worldwide (1). It is estimated to affect 1% of the population in most countries globally, with about 71 million chronic infections and about 400.000 deaths annually (2). The Eastern Mediterranean and European regions have the highest burden of disease (3).
In Turkey, the seroprevalence of HCV was 0.5% and 1% (4). This means that around 250,000 - 550,000 people over the age of 18 are estimated to be infected with HCV, with the majority of the infected individuals unaware of their condition. Despite its low endemicity, HCV is the second leading cause of liver transplantation in Turkey. If left untreated, HCV is expected to increase the disease burden and mortality steadily over the next 20 years (5-7). To develop effective management and prevention strategies, it is crucial to understand the epidemiological trends, clinical characteristics, and factors influencing disease transmission. Based on the purpose of these factors, this study has examined the demographic, clinical, and virological profiles of patients diagnosed with hepatitis C from 2003 to 2023 over two decades in a central hospital in the southern region of Turkey.
2. Objectives
It aimed to highlight the importance of continuous surveillance, targeted prevention efforts, and individualised management strategies to reduce the burden of hepatitis C and improve patient outcomes.
3. Methods
This study reviewed adults admitted to Balcalı Hospital, Department of Infectious Diseases, who tested positive for anti-HCV and HCV RNA. Balcalı Hospital is located in Adana, Turkey, and is affiliated with Çukurova University, a prominent educational and research institution in southern Turkey. The demographic characteristics, admission dates, hepatitis B virus (HBV) coinfection status, HCV genotypes, HCV RNA values, and liver enzymes were recorded.
3.1. Inclusion Criteria
1. Patients aged 18 and older;
2. Patients diagnosed with HCV from January 2003 to January 2023;
3. Anti-HCV positive patients whose initial HCV RNA levels were measured.
3.2. Exclusion Criteria
1. Patients under the age of 18;
2. Patients with undetectable initial HCV RNA for any reason.
An anti-HCV antibody test was performed using a chemiluminescent microparticle enzyme immunoassay (CMIA) (Architect, Anti-HCV, Abbott, USA) following manufacturer’s guidelines.
Hepatitis C virus RNA quantitation in serum was performed using a real-time polymerase chain reaction (PCR) test kit (COBAS AmpliPrep/COBAS TaqMan HCV real-time PCR, Roche diagnostics, Germany). For HCV genotype determination, the HCV Genotype Plus real-TM (Sacace Biotechnologies, Italy) kit was used.
3.3. Statistical Analysis
The data in the study were presented according to the following conventions: Categorical data were presented as number (n) and percentage (%), while numerical data were displayed as mean ± standard deviation or median (Q1 - Q3). The Fisher’s exact test was used to compare categorical measurements between groups. In addition, the Kolmogorov-Smirnov test was applied to assess whether the numerical measurements met the assumption of normal distribution. Moreover, the Kruskal-Walli’s test was utilized for a general comparison of numerical measurements that did not demonstrate a normal distribution among more than two groups. In cases where the general comparison was significant, Dunn’s test was conducted for pairwise group comparisons, and lettering was assigned according to the corrected p-values. The statistical analysis of the data was performed using the IBM SPSS 20.0 package. All tests were conducted with a statistical significance level of 0.05.
4. Results
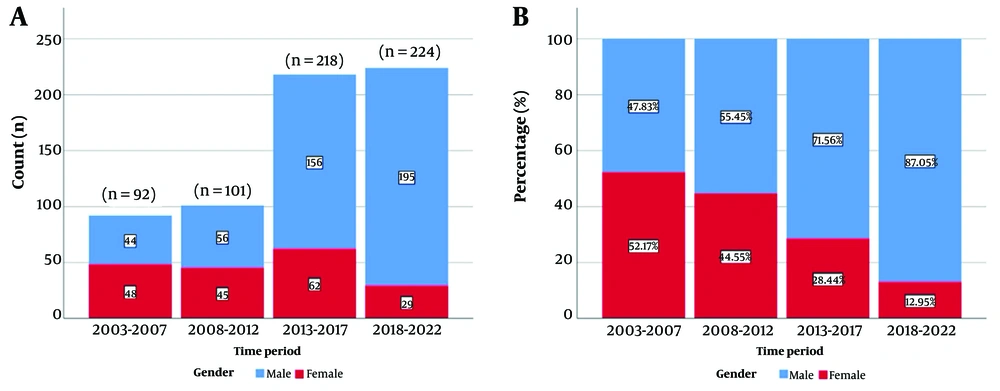
A total of 635 patients were studied from 2003 to 2023. The patient distribution was analyzed over four periods, and the number of patients increased gradually over time (Figure 1).
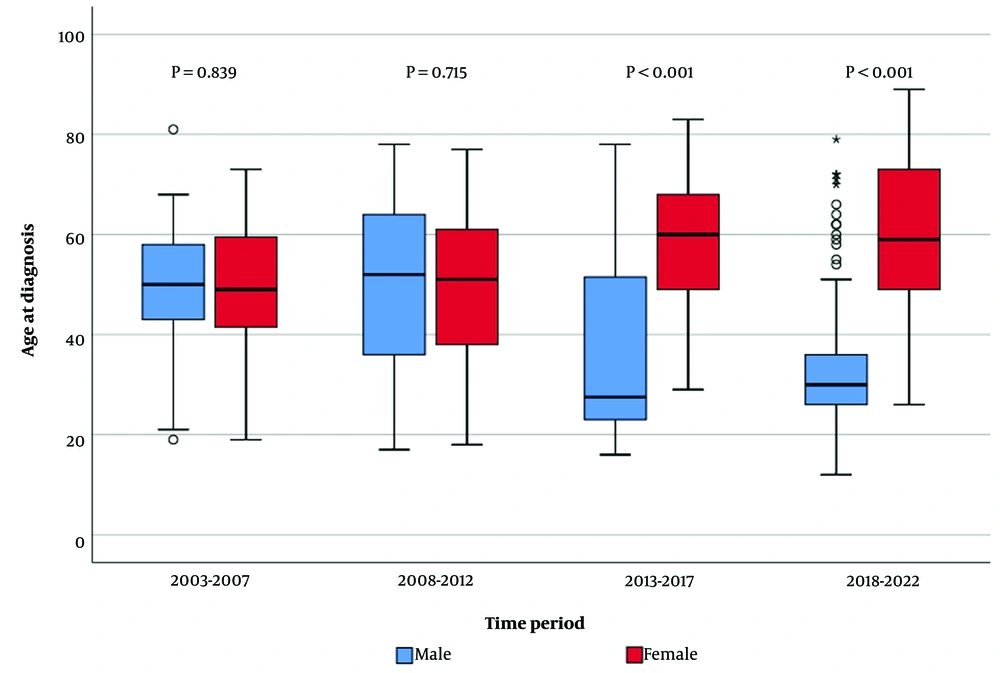
The mean age at diagnosis was 42.3 ± 17.4 years (range = 12-89), and no statistically significant difference was found between 2003 - 2007 and 2008-2012 (P = 0.776). These years exhibited a higher age at diagnosis compared to 2013 - 2017 and 2018 - 2022 periods, indicating a decreasing trend in the age at diagnosis over the years (Figure 2).
Out of the patients, 71% were male. The gender distribution of patients diagnosed with HCV was analysed by year. It was concluded that the proportion of male patients increased over the years, while the proportion of female patients gradually decreased (Figure 2). Gender ratios were found to be statistically different across years (P < 0.001).
In 55.4% of the patients, the transmission route was unknown. It was found that the cause of transmission was unknown at a high rate in 2012 and the previous period, while the rate of unknown transmission was lower in the following years. The most common transmission route was intravenous drug use (IVDU), accounting for 40.3%. The rate of IVDU was 46.3% between 2013 and 2017 and 68.3% between 2018 and 2022 (Table 1).
| Transmission Routes | Time Period | P-Value | |||
|---|---|---|---|---|---|
| 2003 - 2007 | 2008 - 2012 | 2013 - 2017 | 2018 - 2022 | ||
| Unknown | 91 (98.9) | 98 (97.0) | 102 (46.8) | 61 (27.2) | <0.001 |
| IVDU | 0 (0.0) | 2 (2.0) | 101 (46.3) | 153 (68.3) | |
| Hemodialysis | 0 (0.0) | 0(0.0) | 9 (4.1) | 5 (2.2) | |
| Unsafe procedure | 1 (1.1) | 1 (1.1) | 6 (2.7) | 5 (2.2) | |
| Total (n) | 92 | 101 | 218 | 224 | - |
Abbreviation: IVDU, intravenous drug use.
a Unsafe procedure: Unsafe tattooing or piercing, or receiving medical care with improperly sterilized or reused medical equipment, particularly in settings with inadequate infection control practices.
b Values are expressed as No. (%).
The proportion of patients with a prison history was 9.1%. Upon analyzing the prison history, it was observed that none of the patients had a prison history in 2003 - 2007 and 2008 - 2012. In 2013 - 2017, 5.2% of the patients had a prison history, while in 2018 - 2022, 24.6% had a prison history. The rate was significantly higher between 2018 and 2022 than in the other year intervals (P < 0.001).
The mean values of HCV RNA, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels are shown in Table 2. The median value of HCV RNA was higher in the 2018 - 2022 year interval than in the other years. Aspartate aminotransferase levels were similar across the groups (P = 0.148), while ALT levels showed a significant difference (P = 0.001) (Table 2).
| Variables | Time Period | P-Value | ||||
|---|---|---|---|---|---|---|
| 2003 - 2023 | 2003 - 2007 | 2008 - 2012 | 2013 - 2017 | 2018 - 2022 | ||
| HCV RNA; (IU/mL) | 970000 | 906500 A (179193 - 2370000) | 543000 A (80000 - 2400000) | 845041 A (213000 - 3360000) | 1851000 B (265000 - 7457000) | < 0.001 |
| AST; (IU/L) | 35 | 35 (25 - 58) | 36 (28 - 57) | 36 (28 - 51) | 34 (25 - 46) | 0.148 |
| ALT; (IU/L) | 40 | 33 (24 - 54) A | 34 (27 - 50) A | 43 (33 - 70) B | 43 (27 - 66) AB | 0.001 |
Abbreviations: HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
a For the variable of interest, the same capital letters indicate that the groups are similar, whereas different capital letters indicate a statistically significant difference.
b Results are presented as median (Q1 - Q3).
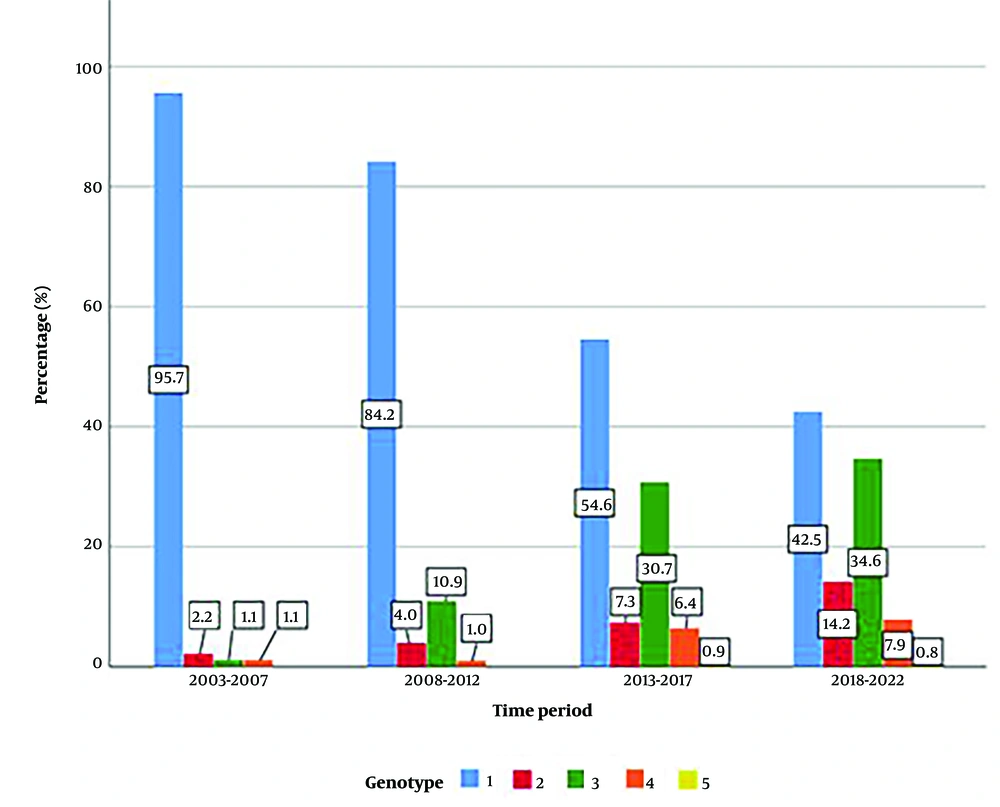
The distribution of genotypes was found to be statistically different across time periods (P < 0.001). Genotype 1 was the most prevalent between 2003 and 2007, with no instances of genotype 5 observed. This trend continued in 2008 - 2012. However, the rates of genotype 3, genotype 4, and genotype 5 were higher in the last two time periods than in the previous years (Figure 3).
5. Discussion
The findings of this study offer insightful information about the changing landscape of hepatitis C diagnosis, demographics, transmission routes, and disease characteristics from 2003 to 2023. Understanding these trends is crucial for informing public health strategies and clinical management approaches. In this discussion, we will delve into the implications of the key findings and their broader significance.
A notable trend observed in this study is the gradual increase in the number of diagnosed hepatitis C cases over the four periods analyzed. The number of patients increased gradually over time, particularly after 2012. This rise could be attributed to several factors, including increased awareness, improved screening programs, and changes in risk behaviors within the population. In a study published in 2012, it was reported that the prevalence of anti-HCV was between 0.5% and 1% in our country (8). Accordingly, it is estimated that approximately 250,000 - 550,000 adults are infected with HCV, and the majority of them are unaware of their condition. A study published in 2014 estimated that 514,000 people (0.7% of the population) were infected with HCV in Turkey as of 2013. According to the same study, it was estimated that 81,300 (16%) people were infected with HCV, with 5,500 (1.1%) newly diagnosed cases and 4,200 (0.8%) patients receiving annual treatment. While the number of individuals with hepatocellular carcinoma (HCC) was 2,230 in 2013, it is estimated to increase by 70% in 2030. Similarly, liver-related deaths are expected to increase by 70% above the 2013 level in 2020 and by 60% and 40% in decompensated and compensated cirrhosis, respectively (6, 9-11). In our study, it was suggested that the increase in the number of cases might be due to the widespread use of screening programs, increased awareness, and the rise in the number of diagnosed patients (6). In the United States, the incidence of new hepatitis C infections reported to the Centers for Disease Control and Prevention (CDC) in 2018 was four times higher than that in 2009. In addition, 2018 marked a decade of increasing incidence of new hepatitis C infections in those aged between 20 and 30, with IVDU representing the predominant transmission route (12). Consequently, the CDC updated screening recommendations and offered universal HCV screening among adults and pregnant women. The findings underscore the importance of ongoing surveillance and intervention efforts to effectively address the growing burden of hepatitis C (13).
While the mean age at diagnosis remained relatively stable between 2003 - 2007 and 2008 - 2012, a notable decline was noted in subsequent years. This shift may reflect changing patterns of transmission and evolving risk profiles among different age groups. The gender distribution of hepatitis C patients also showed notable changes over time, with a higher proportion of male patients observed across all periods. This finding aligns with existing literature, suggesting a higher prevalence of HCV among males, possibly due to differential exposure to risk factors, such as IVDU and occupational hazards. The increasing proportion of male patients over the years underscores the need for targeted interventions tailored to address gender-specific risk factors and access barriers to testing and treatment. According to the 2020 European CDC Annual Epidemiological Report, hepatitis C was more commonly reported among men than women, with a male-to-female ratio of 2.2:1. The most affected age group among males was 35 - 44 years, while among females, it was 25 - 44 years (10). In our study, the mean age was 42.3, and the male-to-female ratio was 2.4:1. In a study in Turkey, the mean age was reported to be 52, and 52.4% of the study population was female. This study was conducted in Istanbul, and different results may be due to sociocultural differences with our region (11). In a study cohort conducted in Italy, 51.0% of patients were female, with a mean age of 47.5 years for female patients and 50.5 years for male patients (14). In our study, it was concluded that the proportion of male patients increased over the years, and the proportion of female patients gradually decreased (Figure 2). Gender ratios were found to be statistically different according to years (P < 0.001).
In 55.4% of the patients, the transmission route was unknown, and the cause of transmission was unknown at a high rate in 2012 and the previous period, while this rate was lower in the following years. The most frequently reported transmission route was IVDU, accounting for 40.3% of cases. The rate of IVDU was 46.3% between 2013 and 2017 and 68.3% between 2018 and 2022 (Table 1). In the ECDC Annual Epidemiological Report, the transmission route was reported for just 31% of cases, and similar to our study, the most commonly reported transmission route was IVDU, accounting for 59% of cases with complete information on transmission status (10). Initially, IVDU emerged as a significant transmission route in industrialized countries during the 1960s. However, it has since spread to affect numerous countries worldwide, including both urban and rural areas. In the United States, for instance, IVDU has recently emerged as a prominent issue, particularly in certain regions. In our country, the prevalence of HCV in patients receiving treatment in addiction treatment centers is around 50% (15). In countries like ours, where harm reduction policies are not yet effectively implemented, controlling HCV transmission among IVDUs remains a challenge. The implementation of harm reduction policies, needle and syringe exchange programs, and the development of opioid substitution treatments have significantly contributed to reducing the prevalence of infectious diseases in industrialized countries. For instance, in France, the prevalence of HCV among IVDU aged 30 years or younger decreased from 44% in 2004 to 9% in 2011 (16). The study also shed light on the shifting patterns of transmission routes, with IVDU emerging as the most common transmission route. The substantial increase in the prevalence of IVDU as a transmission route, particularly in the later years studied, highlights the urgent need for inclusive harm reduction strategies, including needle exchange programs, opioid replacement therapy, and access to evidence-based addiction treatment services. Addressing the social determinants of health underlying substance use disorders is crucial for preventing new hepatitis C infections and reducing transmission rates within vulnerable populations.
The rise in the proportion of patients with a history of incarceration is another concerning trend identified in this study, particularly in the later years analyzed. All 9.1% of patients had a prison history; this rate was higher between 2018 and 2022 than the other year intervals (P < 0.001).
The higher prevalence of HCV among individuals with a history of incarceration underscores the importance of targeted screening and treatment initiatives within correctional settings. Implementing comprehensive hepatitis C care programs in prisons can not only improve health outcomes for incarcerated individuals but also contribute to broader public health goals by reducing transmission rates in the community upon release. In Larney’s study, the prevalence of anti-HCV in prisoners was reported to be 26%, while it could be up to 64% in IVDU prisoners. In the same study, while anti-HCV was 1.4% in the general population, anti-HCV was found to be 16.4% in IVDU individuals (17). In Çabalak’s study, the prevalence of IVDU among prisoners with a high prevalence of HCV was 37.6%. They suggested that this might be due to the low number of patients and geographical region differences (16). In Zampino et al.’s study, the prevalence of anti-HCV in convicted patients was reported to range from 3% to 38% according to geographic region, IVDU, age, and prisoners’ history (17). Preventing the spread of infectious diseases among prisoners by early diagnosis for the first time in a penal institution or already in a penal institution HBV, HCV, and human immunodeficiency virus (HIV) screening tests for all detainees. It is important to have it conducted voluntarily. This increase may be the result of increased screening of high-risk groups within the scope of hepatitis elimination programs in Turkey.
The analysis of disease characteristics, including HCV RNA levels, liver enzyme levels, and genotype distribution, provides valuable insights into the clinical profile of hepatitis C patients over time. The observed differences in HCV RNA levels and genotype distribution across the study periods may reflect changes in disease epidemiology, advancements in diagnostic technologies, or evolving treatment landscapes. The distribution of genotypes was found to be statistically different across time periods (P < 0.001). After 2013, almost half of the cases were infected with strains other than genotype 1. The increase in genotypes 2 and 3 was particularly notable. In 2014, a study conducted in the Çukurova region also found an increase in genotypes 2 and 3 and noted that the rate of IVDU in these cases was high (18).
Overall, the key findings reveal a gradual increase in diagnosed cases, a shift towards a younger age at diagnosis over time, and a notable rise in IVDU as a transmission route. All of these underscores the dynamic nature of hepatitis C epidemiology and highlight the importance of ongoing surveillance, prevention, and treatment efforts.
The study's limitations included its retrospective nature and single-center design, potentially limiting broader applicability. Selection bias towards more severe cases and variations in data completeness over time could affect the reliability of findings. In addition, the significant proportion of patients with unknown transmission routes and changes in diagnostic practices over the study period further complicate the interpretation of trends.