1. Background
Non-communicable diseases have become a major challenge for health systems worldwide and are responsible for an increasing number of premature deaths and preventable morbidity and disability (1). Globalization, change in life style, reduced physical activity, and unhealthy diet are among the causing factors for the increase in the prevalence and burden of non-communicable diseases (2).
Nonalcoholic fatty liver disease (NAFLD) is a common non-communicable disease affecting 20% to 30% of adult populations in developed countries (3). Furthermore, NAFLD is generally associated with obesity, sedentary life style, and metabolic syndrome (4). It includes a wide range of liver pathology from mild steatosis to severe hepatic fibrosis and cirrhosis (5). Thus, diagnosis of NAFLD could at times be problematic (6). Even when the diagnosis is achieved, treatment is not easily established, as there are no approved drugs for treatment. Several treatment protocols are suggested and applied by clinicians and researchers, yet there is no consensus among experts and clinicians on medical treatment (7). Generally, a combination of modified diet and exercise is recommended for treatment (8).
Some researchers have shown that a high antioxidants and anti-inflammatory diet could be effective in NAFLD treatment (9). Food bioactive compounds are among the suggested therapeutic approaches for NAFLD (10). In fact, some anti-oxidants, anti-inflammatory, and insulin sensitizer dietary supplements are believed to modulate the activation of genes involved in lipogenesis, fibrogenesis, lipid peroxidation, and inflammation (9).
Iron chelating agents, such as green tea extracts, are suggested by some researchers to have beneficial effects in animal and human studies (11). These effects are observed on obesity, total and visceral body fat, insulin resistance, serum cholesterol, and different degrees of liver steatosis. Steatosis is reduced by decreased lipids and carbohydrates absorption and inhibited adipose tissue turn over in both hepatic and adipose tissues. Antioxidant and anti-inflammatory characteristics of the active agents result in inhibited steatohepatitis (12).
Despite the findings of a few studies evaluating the effect of green tea, there are controversies regarding the effects of green tea extract on NAFLD. More studies are needed to provide enough evidence on the probable effect of green tea on preventing the development and/or progression of NAFLD (9).
In this study, the authors evaluated the effect of green tea extract as a general antioxidant and iron chelating agent on liver function, anthropometric measures, and Iron markers in a randomized double blind controlled trial on patients with NAFLD.
2. Methods
The study protocol of this double-blind randomized controlled trial was approved by the ethical committee of Iran University of Medical Sciences. The study was also registered on the Iranian registry of clinical trials (IRCT), as IRCT201404132365N8.
The researchers evaluated 108 known cases of NAFLD for inclusion and exclusion criteria, from which 67 cases entered the study. The inclusion criteria were confirmed NAFLD diagnosis by a gastroenterologist with ultrasonography, liver biopsy or liver Fibroscan, age of 18 or older, and willingness to participate in the study. The exclusion criteria were Iron deficiency anemia, allergy of green tea, history of alcohol consumption (more than 20 grams daily), other liver disorders (viral hepatitis, auto immune hepatitis, celiac, Wilson, and Alpha 1-antitrypsin deficiency), pregnancy, and lactation. Participants were also supposed to be excluded during the study if they showed allergy or other side effects, became pregnant, and consumed less than 80% of the supplements they received or did not wish to continue the study.
The participants were randomly assigned to 2 groups based on a list already generated using a random number sequence. Only the main researcher had access to this list and could detect if a certain participant was receiving supplements or placebo.
Patients in the intervention group received 550 milligrams of green tea tablets daily in divided doses, as well as nutritional education and consultation for weight loss with low calorie diet and life style change recommendation (minimum 2 to 3 sessions of 30 to 60 minutes of aerobic exercise weekly) for 3 months. The control group received the same protocol with green tea replaced with identical placebo capsules with starch composition.
Anthropometric evaluation, body composition, food intake for energy, nutritional agents and Iron, liver enzymes, fasting blood sugar, insulin, hemoglobin, TIBC, ferritin, transferrin, serum Iron, transferrin saturation, total antioxidant capacity, and malondialdehyde were evaluated at the beginning and end of the study. Biologic sample was acquired for mRNA extraction and cDNA synthesis using reverse transcriptase.
Statistical analysis was performed using SPSS version 19. Mean, Standard Deviation (SD), and percentage were used for describing the data. Normality of the data was evaluated by Shapiro-Wilk’s Test. T test and Mann-Whitney U test were used for comparing the groups.
3. Results
After primary evaluation, 67 participants in the 2 groups (33 in the intervention and 34 in the control group) were studied. Mean age of participants in the intervention and control group was 41 and 39.5, respectively (P value of 0.61). There were 18 female participants in the intervention and 22 in the control group. (P value of 0.85). In each group, 7 people had diabetes (P value: 0.73). There were no statistical differences in other baseline characteristics (Table 1).
| Variables | Control | Intervention | P Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| TAC | 6.88 | 2.47 | 7.18 | 2.50 | 0.655 |
| Hemoglobin | 14.26 | 1.28 | 14.61 | 1.36 | 0.345 |
| Iron | 68.74 | 29.60 | 83.43 | 33.64 | 0.115 |
| TIBC | 346.16 | 39.43 | 333.57 | 27.28 | 0.223 |
| Ferritin | 91.12 | 96.76 | 136.68 | 135.16 | 0.159 |
| Transferrin | 260.87 | 56.76 | 261.21 | 50.75 | 0.984 |
| FBS | 110.88 | 47.22 | 113.55 | 44.40 | 0.835 |
| Ins | 16.49 | 10.23 | 18.75 | 12.97 | 0.512 |
| Cholesterol | 193.31 | 36.87 | 194.45 | 39.63 | 0.914 |
| TG | 132.75 | 66.37 | 152.23 | 65.30 | 0.291 |
| HDL | 47.88 | 8.20 | 44.68 | 7.43 | 0.150 |
| LDL | 112.44 | 33.56 | 112.45 | 23.56 | 0.998 |
| ALT | 31.16 | 21.33 | 32.41 | 29.43 | 0.857 |
| AST | 23.72 | 12.52 | 25.36 | 16.83 | 0.682 |
| HOMA | 10.02 | 9.03 | 13.33 | 12.03 | 0.244 |
| Weight | 129.64 | 32.93 | 116.75 | 30.83 | 0.151 |
| BMI | 44.31 | 6.68 | 43.06 | 7.37 | 0.540 |
| Fat Mass | 55.90 | 14.34 | 51.83 | 15.11 | 0.344 |
| MDA | 58.27 | 54.98 | 89.73 | 84.32 | 0.150 |
| Iron intake | 10.9 | 3.69 | 9.7 | 3.57 | 0.31 |
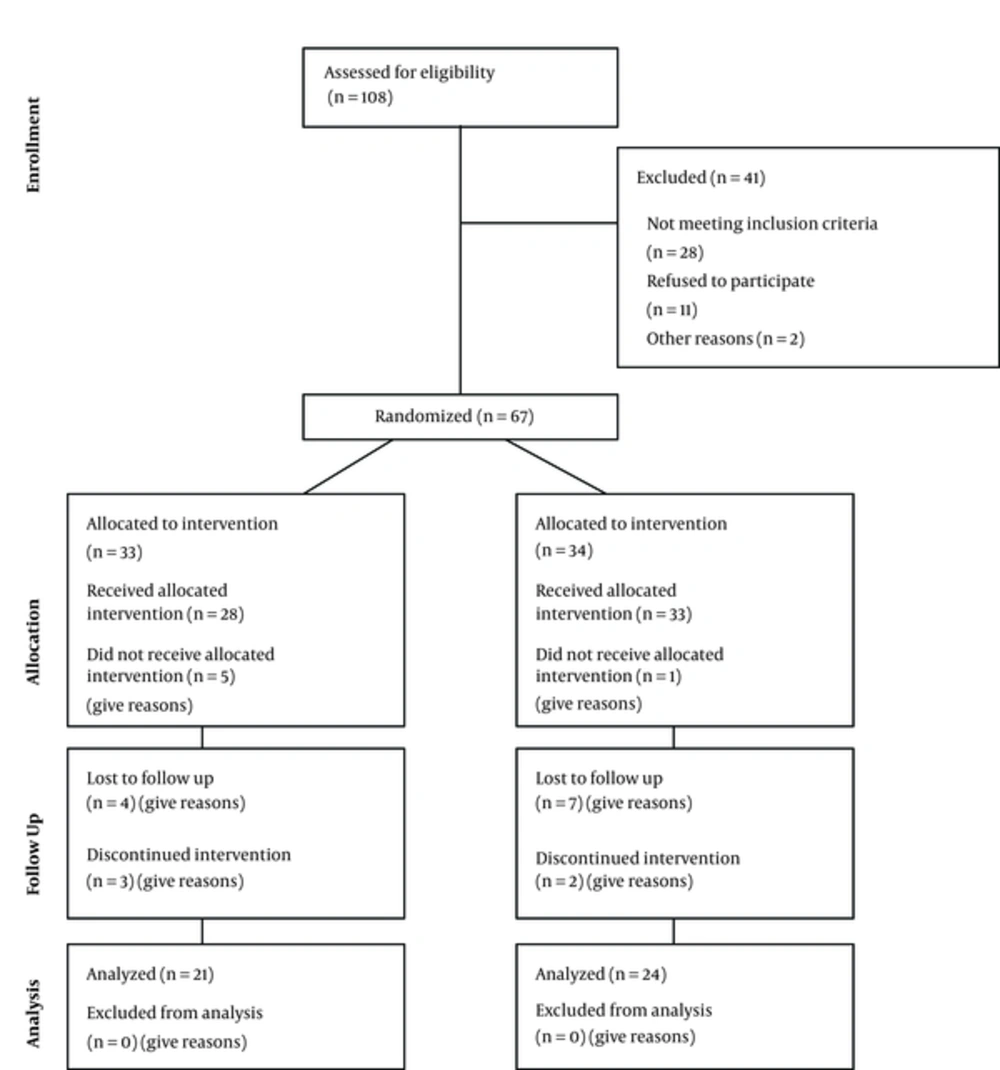
Each participant was followed for 3 months and finally 45 participants (21 in the intervention and 24 in the placebo group) completed the follow-up. Two of the control group and 3 of the intervention group participants discontinued due to assumed side effects. The reasons for discontinuation were immigration, other medical problems, side effects, and losing their interest in the study. Details are presented in the participant’s flow diagram (Figure 1).
Table 2 shows the mean change of the study variables after 3 months in each group. The difference in the mean change of the 2 groups was statistically significant for BMI, AST, and FBS, in contrast to MCV, MCHC, TIBC, Hb, Ferritin, ALT, HOMA, and weight.
| Variables | Control | Intervention | P Value | ||
|---|---|---|---|---|---|
| Mean | Std. Deviation | Mean | Std. Deviation | ||
| TAC | 2.95 | 1.87 | 2.58 | 2.43 | 0.597 |
| HB | 0.09 | 0.90 | -0.13 | 0.75 | 0.431 |
| Iron | 7.12 | 39.01 | -8.87 | 33.62 | 0.218 |
| TIBC | -11.00 | 58.95 | 2.50 | 43.27 | 0.193a |
| Ferritin | 2.11 | 24.76 | -28.46 | 74.89 | 0.496a |
| Transfer | -10.73 | 43.66 | -12.47 | 37.39 | 0.914 |
| FBS | 6.35 | 27.32 | -0.89 | 34.80 | 0.019a |
| Ins | 0.62 | 9.37 | -0.58 | 8.78 | 0.708 |
| Cholesterol | 1.42 | 31.43 | -12.67 | 43.17 | 0.262 |
| TG | 4.58 | 44.72 | -18.89 | 56.08 | 0.167 |
| HDL | -3.37 | 6.28 | -2.39 | 9.33 | 0.709 |
| LDL | -3.84 | 29.14 | -6.50 | 25.88 | 0.771 |
| Alt | -3.50 | 21.85 | -12.17 | 19.17 | 0.079a |
| AST | -0.22 | 14.63 | -6.78 | 13.42 | 0.037a |
| HOMA | -3.17 | 9.20 | -3.43 | 9.42 | 0.802a |
| Weight | -1.83 | 5.15 | -5.27 | 6.45 | 0.143a |
| BMI | -0.25 | 2.02 | -1.88 | 1.86 | 0.037 |
| Fat Mass | -2.90 | 3.19 | -4.28 | 4.51 | 0.713a |
| MDA f | 21.98 | 60.97 | -0.89 | 67.09 | 0.278 |
aMann-Whitney U test.
4. Discussion
The current study evaluated the effect of green tea extract on various aspects of NAFLD pathology and treatment. The researchers evaluated liver function tests and observed better AST results in the intervention group compared to controls. The change in ALT was similar in direction, yet not statistically significant. In a similar study on a group of Iranian patients, significant changes were observed in ALT and AST (13). The study of Sakato et al. also showed significant changes in ALT but not AST (14). Considering the high prevalence of obesity in the current sample and the majority of grade 2 and 3 fatty liver, more prolonged interventions may be needed to determine significant changes in both liver function tests.
Among anthropometric parameters evaluated in this study, the changes in BMI was more pronounced in green tea consumers than the control group, who received routine treatment. Changes in other anthropometric parameters, such as body weight, fat mass, and fat proportion did not present significant differences. Nagao et al. showed similar effects of green tea on different anthropometric parameters (15). Other researchers have also observed similar results with longer follow up durations (14, 16). Sakata et al. showed a three-fold increase in weight loss in patients with NAFLD, although it was not statistically significant (14). The current study showed a significant reduction in green tea group compared to the control group although the quantity was less.
Hemoglobin, Iron, Transferrin, and Ferritin were measured as markers of iron level. At the end of the study, the change was more dominant in the intervention group than the controls, while the difference was not statistically significant. The iron chelating activity of green tea was widely discussed in other studies (17) and researchers have highlighted positive effects (18).
The researchers did not observe statistically significant differences in antioxidant markers in this study, while some other researchers have documented such effects in animal (19) and human studies (20, 21). The study of Basu et al. showed change in MDA in 35 fat patients with metabolic syndrome with average BMI equal to 35. Also, the administered dose is higher in their study, compared to the current work. The fact that the current patients had much higher BMI average (44) might be the reason for the changes observed in the current study sample (20).
The difference in metabolic marker change between the 2 groups were present yet not statistically significant, except for FBS. Researchers have shown the positive effect of green tea in reducing insulin resistance, yet we could not document such change in the current study (22).
While the iron chelating activity of green tea has been widely discussed, (17) the researchers did not observe differences in iron markers. It seems that the therapeutic effects of green tea are generally explained through anti-oxidant features (23). These features are being studied in a variety of disease, such as Parkinson’s and Alzheimer’s disease (24) and other neurodegenerative disorders (17), cardiovascular disease, and malignancies among others.
Although there is supporting literature on health promoting effects of green tea in vitro and in animal studies (25, 26), the researchers need more evidence from human research before it could be considered as a widely accepted therapeutic agent (27). Also, the researchers need to gain a detailed understanding of its mechanisms at the molecular level and the pathways through which it affects fat accumulation, oxidative stress, and inflammation (10).
The researchers observed positive effects from green tea mainly in anthropometrics, liver enzyme, and metabolic indicators. This could be due to the short duration of the study or the limited sample size. Also, the green tea extract dose was higher in most of the other studies. The difference between the intervention and control group might not be highlighted due to the effectiveness of routine treatments that both groups received.