1. Background
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a comprehensive term that refers to hepatic steatosis associated with obesity or overweight, diabetes mellitus, or the presence of at least two metabolic abnormalities. These abnormalities include increased waist circumference (WC), hypertension, prediabetes, hypertriglyceridemia, and reduced levels of high-density lipoprotein cholesterol (HDL-C) (1). Hepatic steatosis may induce hepatic inflammation and fibrogenesis, which can advance to liver cirrhosis, liver failure, and hepatocellular carcinoma (2). In the last few decades, rapid changes in lifestyles and dietary habits increased the prevalence of MASLD to 32.4% worldwide and 33% in Iran (3, 4). However, the health system still lacks a screening program to detect individuals at risk of MASLD and related complications (5). Liver biopsy is still the gold standard for the diagnosis of liver steatosis and fibrosis, but non-invasive techniques are commonly used for diagnostic purposes in clinical settings (6). Transient elastography (TE) is an accurate alternative to liver biopsy for diagnosing and grading hepatic steatosis and fibrosis (6, 7). However, the expenses of its use restrict its application in research settings.
Several factors contribute to the development and progression of MASLD, including physical inactivity, obesity, visceral adipose tissue (VAT), insulin resistance, dyslipidemia, and liver inflammation (8-10). Visceral fat, compared to subcutaneous fat, plays a significant role in the development and progression of MASLD (11). Computed tomography (CT) and Magnetic Resonance imaging (MRI) are valuable tools for assessing fat distribution and measuring VAT. However, their high costs, time consumption, and potential radiation exposure present significant drawbacks. As a result, there is a growing interest in utilizing surrogate markers as alternative methods for evaluating fat distribution (12). Waist circumference, waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) are anthropometric parameters introduced for abdominal obesity; however, they cannot differentiate visceral from subcutaneous fat (13, 14). Therefore, developing an index that estimates the measure of VAT was necessary.
Lipid accumulation product (LAP) is a classic marker that has shown good performance in predicting visceral fat function, metabolic diseases, and MASLD; however, its role in predicting liver fibrosis remains relatively obscure (15-17). Furthermore, substantial evidence supports that the Visceral Adiposity Index (VAI) has considerable value in predicting metabolic diseases and MASLD; however, there is still controversy regarding the use of VAI as a predictor of liver fibrosis (18-21). Xia et al. (22) developed a Chinese version of the Visceral Adiposity Index (CVAI) to estimate VAT in the Chinese population using age, Body Mass Index (BMI), WC, triglycerides (TG), and HDL-C. Previous studies indicated that CVAI is significantly associated with diabetes, hypertension, and new-onset MI (23-25). Additionally, CVAI plays a significant role in the development of MASLD in healthy Chinese individuals (12). It also demonstrated good diagnostic value for distinguishing MASLD patients from healthy subjects, supporting its use as a screening tool (26).
The role of CVAI in predicting and diagnosing liver steatosis has been investigated in the Chinese population (12, 26). However, it has not been explored in other Asian populations. Additionally, the value of CVAI in predicting and diagnosing significant fibrosis remains to be elucidated.
2. Objectives
This study aimed to explore the value of CVAI in predicting, grading, and diagnosing hepatic steatosis and fibrosis compared to classic visceral fat markers, such as VAI and LAP.
3. Methods
3.1. Design and Participants
The participants of this cross-sectional study were randomly selected from patients attending a nutrition clinic in the south of Iran (Bandar Abbas city) during 2023. The required sample size for comparing two areas under the receiver operating characteristic curve (AUROC) was calculated to be 274 individuals, with a significance level (α) of 0.05 and a power (β) of 0.2, using GraphPad statistical software. The sample size determination was based on findings by Shen et al. (26), which reported an AUROC of 0.819 for CVAI and 0.772 for VAI, along with a correlation coefficient of 0.85 in both MASLD and healthy groups. Ultimately, 300 subjects over the age of 18 were included, of whom 27 were excluded due to alcohol consumption more than twice a week, any history of infectious or autoimmune hepatitis, congenital hepatic disease, use of anti-epileptics, corticosteroids, methotrexate, tamoxifen, or chemotherapeutic agents, as well as pregnancy or cancer within the last six months. This study was approved by the Ethics Committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1403.105) and adhered to the 1964 Declaration of Helsinki and its later amendments. All participants provided written informed consent.
3.2. Data Collection
Demographic data, medical history, and physical examination findings were collected via a checklist by an expert nutritionist at the nutrition clinic. Weight was measured to the nearest 0.1 kilograms using a clinical scale (SECA 704; Hamburg, Germany), with participants wearing minimal light clothing and standing barefoot. Height was measured with a standard stadiometer, also without footwear. Body Mass Index was calculated using the formula: BMI = weight (kg) ÷ height (m2). Waist circumference was measured at the midpoint between the last rib and the iliac crest. Fasting blood sugar (FBS), TG, total cholesterol, LDL-C, HDL-C, aspartate transaminase (AST), and alanine transaminase (ALT) were measured after a 12-hour fast using quantitative diagnostic kits. FibroScan® (Echosens 504, Paris, France) was used to measure the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM). The appropriate probe (M or XL) was selected based on the skin-liver capsule distance. The median of ten measurements with IQR/M < 30% was considered a reliable LSM for each patient (27). Compared to liver biopsy, FibroScan demonstrated an AUROC of 0.82, 0.86, and 0.87 for diagnosing F ≥ F2, F ≥ F3, and F ≥ F4, respectively (28).
Abdominal obesity was defined as a WC of 102 cm or more in males and 88 cm or more in females. Hypertriglyceridemia was defined as TG levels of 150 mg/dL or more, or a history of taking lipid-lowering agents. Additionally, males with HDL-C levels of 40 mg/dL or lower, and females with HDL-C levels of 50 mg/dL or lower, were categorized as having decreased HDL-C levels. Diabetes was attributed to participants with FBS levels of 126 mg/dL or higher, or a history of taking antihyperglycemic drugs (29, 30). Values of 238, 260, and 292 dB/m were used for distinguishing S1, S2, and S3, respectively, for steatosis grading. For fibrosis staging, values of 6.2, 7.6, 8.8, and 11.8 kPa were used to determine F1, F2, F3, and F4, respectively (31). Significant fibrosis was defined as F ≥ F2 (32).
Lipid accumulation product was calculated based on Kahn’s study (15). Visceral Adiposity Index was calculated according to Amato et al.'s study (18). Chinese Visceral Adiposity Index (CVAI) was calculated according to Xia et al.'s formula (22). The formulas are as follows:
- Male: (1) LAP = [WC (cm) – 65] × [TG (mM)]; (2) VAI = [WC (cm)/(39.68 + (1.88 × BMI))] × [TG (mM)]/1.03] × [1.31/HDL-C (mM)]; (3) CVAI = -267.93 + 0.68 × age + 0.03 × BMI (kg/m2) + 4.00 × WC (cm) + 22.00 × Lg TG (mM) - 16.32 × HDL-C (mM)
- Female: (1) LAP = [WC (cm) – 58] × [TG (mM)]; (2) VAI = [WC (cm)/(36.58 + (1.89 × BMI))] × [TG (mM)]/0.81] × [1.52/HDL-C (mM)]; (3) CVAI = -187.32 + 1.71 × age + 4.23 × BMI (Kg/m2) + 1.12 × WC (cm) + 39.76 × Lg TG (mM) - 11.66 × HDL-C (mM)
*'Lg' is the symbol for the logarithm with base ten.
SPSS version 27.0 and MedCalc software version 22.009 were used to conduct all analyses. The Kolmogorov–Smirnov test was used to assess the normality of variables. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were reported as number and percentage. Independent sample t-tests and Mann-Whitney U tests were used to compare the mean of continuous variables between two groups, while the chi-square test was used to compare categorical variables between the groups. Logistic regression was performed to evaluate the predictive value of each visceral fat marker for liver steatosis and significant liver fibrosis. Analysis of variance (ANOVA) was conducted to assess the impact of liver steatosis and fibrosis staging on the values of visceral fat markers. The Welch test was performed if the assumption of equal variances was violated. Tukey and Games-Howell tests were conducted as post hoc tests for classic ANOVA and Welch tests, respectively. The diagnostic accuracy of CVAI and the optimal cut-point value for this index were analyzed by ROC and Youden's Index, respectively. The performance of each visceral marker based on AUROC values is as follows: Fail, 0.5 - 0.6; weak, 0.6 - 0.7; fair, 0.7 - 0.8; good, 0.8 - 0.9; and excellent, 0.9 - 1.0 (33). Statistical analyses used the two-sided test, and a P-value < 0.05 was considered indicative of a significant difference.
4. Results
4.1. Descriptive
In this study, 273 individuals were recruited from patients of a nutrition clinic, with a mean age of 41.74 ± 11.63 years (range: 18 - 73), including 158 (57.9%) males and 115 (42.1%) females. The prevalence of metabolic abnormalities, such as diabetes, hypertriglyceridemia, decreased HDL-C levels, and abdominal obesity, was 66 (24.2%), 142 (52.0%), 142 (52.0%), and 148 (54.2%), respectively. The prevalence of MASLD among participants, by grade, was as follows: Grade I, 37 (13.6%); grade II, 71 (26.0%); grade III, 114 (41.8%). Additionally, the prevalence of liver fibrosis by stage was: Stage I, 44 (16.1%); stage II, 15 (5.5%); stage III, 17 (6.2%); and stage IV, 12 (4.4%). The demographic, anthropometric, and paraclinical data are depicted in Table 1.
| Variables | Overall | Steatosis | Fibrosis | ||||
|---|---|---|---|---|---|---|---|
| Non-steatosis | Steatosis | P-Value | Non-significant | Significant Fibrosis | P-Value | ||
| Age (y) | 41.74 ± 11.63 | 37.68 ± 12.79 | 42.66 ± 11.17 | 0.006 | 41.81 ± 11.426 | 41.20 ± 12.86 | 0.753 |
| Gender | 0.269 | 0.029 | |||||
| Male | 158 (57.9) | 26 (51.0) | 132 (59.5) | 126 (55.0) | 32 (72.7) | ||
| Female | 115 (42.1) | 25 (49.0) | 90 (40.5) | 103 (45.0) | 12 (27.3) | ||
| Diabetes | 66 (24.2) | 11 (21.6) | 55 (24.8) | 0.630 | 49 (21.4) | 17 (38.6) | 0.014 |
| HTG | 142 (52.0) | 14 (27.5) | 128 (57.7) | < 0.001 | 111 (48.5) | 31 (70.5) | 0.008 |
| Low HDL-C | 142 (52.0) | 22 (43.1) | 120 (54.1) | 0.159 | 116 (50.7) | 26 (59.1) | 0.305 |
| Obesity | 148 (54.2) | 12 (23.5) | 136 (61.3) | < 0.001 | 112 (48.9) | 36 (81.8) | < 0.001 |
| BMI (kg/m2) | 28.52 ± 4.62 | 24.71 ± 4.76 | 29.39 ± 4.12 | < 0.001 | 27.77 ± 4.26 | 32.40 ± 4.56 | < 0.001 |
| WC (cm) | 97.12 ± 11.82 | 85.89 ± 12.20 | 99.69 ± 10.14 | < 0.001 | 95.11 ± 10.97 | 107.55 ± 10.75 | < 0.001 |
| ALT (IU/L) | 49.39 ± 45.03 | 33.20 ± 43.55 | 52.80 ± 44.69 | < 0.001 | 42.89 ± 37.56 | 82.16 ± 62.69 | < 0.001 |
| AST (IU/L) | 33.91 ± 25.08 | 25.69 ± 24.86 | 35.65 ± 24.83 | < 0.001 | 29.88 ± 19.48 | 54.27 ± 37.57 | < 0.001 |
| FBS (mg/dL) | 106.72 ± 35.02 | 100.23 ± 20.50 | 108.20 ± 37.44 | 0.150 | 102.90 ± 23.87 | 126.28 ± 65.49 | 0.033 |
| TG (mg/dL) | 159.48 ± 105.90 | 109.78 ± 54.19 | 170.89 ± 111.53 | < 0.001 | 150.96 ± 91.66 | 204.61 ± 155.16 | 0.002 |
| Chol (mg/dL) | 188.82 ± 44.47 | 169.72 ± 35.84 | 193.21 ± 45.16 | < 0.001 | 186.51 ± 44.58 | 201.60 ± 42.56 | 0.023 |
| LDL-C (mg/dL) | 111.44 ± 31.61 | 97.89 ± 30.16 | 114.55 ± 31.18 | 0.001 | 109.42 ± 31.96 | 121.96 ± 28.13 | 0.017 |
| HDL-C (mg/dL) | 43.25 ± 10.76 | 46.38 ± 12.20 | 42.54 ± 10.30 | 0.022 | 43.89 ± 10.83 | 40.33 ± 9.77 | 0.041 |
| LAP | 65.04 ± 53.93 | 32.73 ± 25.24 | 72.43 ± 56.03 | < 0.001 | 57.59 ± 38.99 | 104.24 ± 92.36 | < 0.001 |
| VAI | 2.779 ± 2.249 | 1.788 ± 1.246 | 3.006 ± 2.366 | < 0.001 | 2.583 ± 1.774 | 3.802 ± 3.763 | 0.009 |
| CVAI | 129.16 ± 51.92 | 79.58 ± 55.76 | 140.54 ± 43.78 | < 0.001 | 120.24 ± 48.69 | 174.71 ± 44.41 | < 0.001 |
Abbreviations: HTG, hypertriglyceridemia; BMI, Body Mass Index; WC, waist circumference; ALT, alanine transaminase; AST, aspartate transaminase; FBS, fasting blood sugar; TG, triglyceride; Chol, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; LAP, lipid accumulation product; VAI, Visceral Adiposity Index; CVAI, Chinese Visceral Adiposity Index.
a Values are expressed as No. (%) or mean ± SD.
4.2. Chinese Visceral Adiposity Index in Predicting, Diagnosis, and Grading of Liver Steatosis
Comparison analysis revealed that CVAI values were higher in the liver steatosis group than in the healthy group (140.54 ± 43.78 vs. 79.58 ± 55.76, P < 0.001). The comparison analysis results for VAI and LAP are depicted in Table 1.
In univariate logistic regression, CVAI was significantly associated with liver steatosis (OR = 1.028, CI 95%: 1.019 - 1.037, P < 0.001). After adjustment for age, gender, metabolic disorders, and other visceral markers, each unit increase in CVAI increased the odds of liver steatosis by 3.3% (Adj. OR = 1.033, CI 95%: 1.012 - 1.055, P = 0.002). The logistic regression results for VAI and LAP are shown in Table 2.
| Variables | Steatosis (S ≥ S1) | Significant Fibrosis (F ≥ F2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Model | Multivariate Model | Univariate Model | Multivariate Model | |||||
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Age | 1.039 (1.011 - 1.068) | 0.007 | - | - | 0.996(0.968 - 1.024) | 0.752 | - | - |
| Gender | 0.709 (0.385 - 1.306) | 0.270 | - | - | 0.459 (0.225 - 0.936) | 0.032 | - | - |
| Diabetes | 1.198 (0.575 - 2.494) | 0.630 | - | - | 2.313(1.167 - 4.584) | 0.016 | - | - |
| HTG | 3.599 (1.841 - 7.034) | < 0.001 | - | - | 1.407(0.731 - 2.707) | 0.306 | - | - |
| Obesity | 5.140 (2.549 - 10.361) | < 0.001 | - | - | 4.701(2.094 - 10.554) | < 0.001 | - | - |
| Low HDL-C | 1.551 (0.839 - 2.865) | 0.161 | - | - | 1.407(0.731 - 2.707) | 0.306 | - | - |
| LAP | 1.045 (1.029 - 1.062) | < 0.001 | - | - | 1.016(1.009 - 1.023) | < 0.001 | - | - |
| VAI | 1.756 (1.310 - 2.354) | < 0.001 | - | - | 1.211(1.060 - 1.384) | 0.005 | - | - |
| CVAI | 1.028 (1.019 - 1.037) | < 0.001 | 1.033(1.012 - 1.055) | 0.002 | 1.028(1.019 - 1.038) | < 0.001 | 1.029 (1.011 - 1.047) | 0.001 |
Abbreviations: HTG, hypertriglyceridemia; HDL-C, high-density lipoprotein cholesterol; LAP, lipid accumulation product; VAI, Visceral Adiposity Index; CVAI, Chinese Visceral Adiposity Index.
According to ANOVA, the grade of liver steatosis had a significant impact on CVAI [F (3, 109.29) = 25.695, P < 0.001]. Post hoc analysis indicated that the mean values of CVAI differed significantly between S3-S2 and S1-S0, while the mean values of CVAI in S2-S1 were roughly equal. The mean and SD of VAI and LAP for each grade of liver steatosis are shown in Table 3.
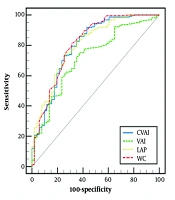
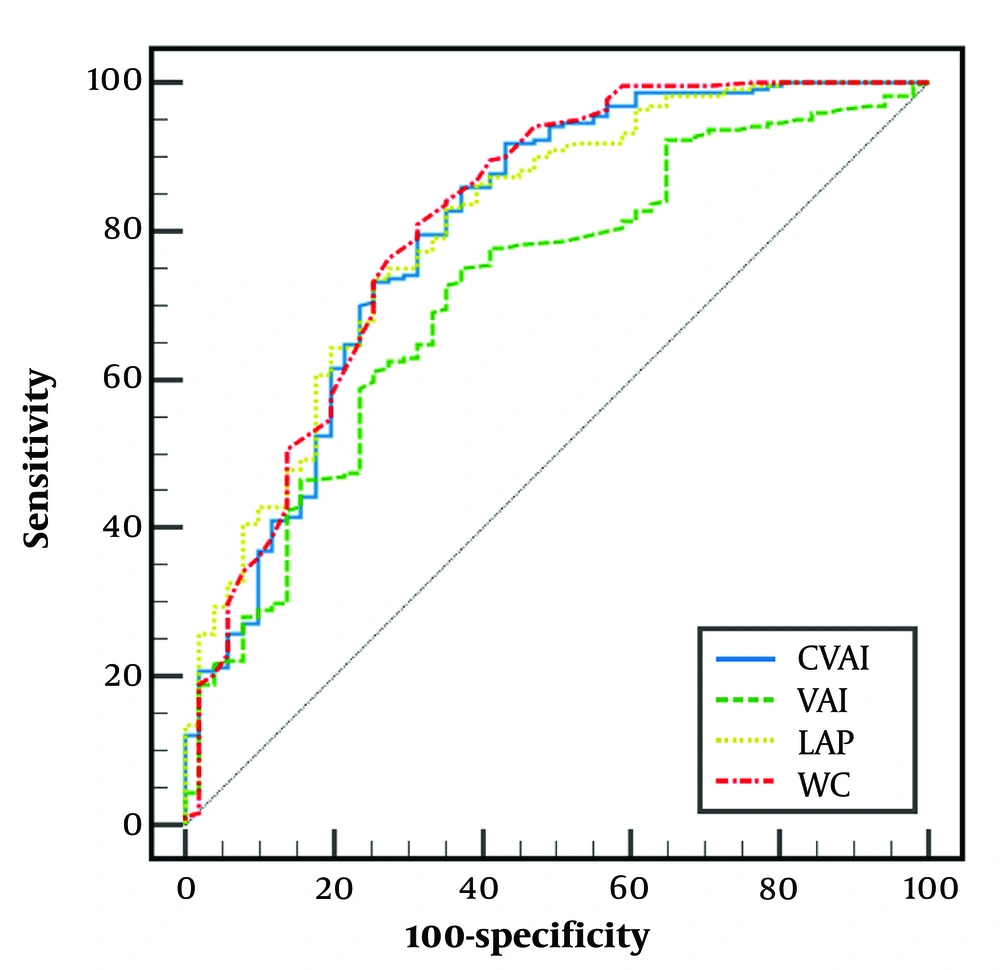
According to ROC analysis, for S ≥ S1, the AUROC of CVAI was 0.800 (95% CI: 0.747 to 0.846) with a cut-point of > 82.62, sensitivity (SN) of 91.86%, and specificity (SP) of 56.86%. The AUROC, cut points, SN, and SP of other visceral fat markers are depicted in Figure 1.
| Variables | Liver Steatosis | Liver Fibrosis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | F | P | F0 | F1 | F2 | F3 | F4 | F | P | |
| CVAI | |||||||||||||
| Mean ± SD | 79.58 ± 55.76 A | 118.10 ± 42.12 B | 131.32 ± 40.32 B | 153.55 ± 42.44 C | 25.6 | < 0.001 | 113.92 ± 48.68 A | 146.69 ± 39.25 B | 170.16 ± 55.97 B | 169.57 ± 43.49 B | 187.68 ± 26.77 B | 17.2 | < 0.001 |
| VAI | |||||||||||||
| Mean ± SD | 1.788 ± 1.246 A | 2.536 ± 2.128 A, B | 2.894 ± 1.919 B | 3.231 ± 2.671 B | 5.2 | 0.002 | 2.520 ± 1.825 | 2.848 ± 1.534 | 4.642 ± 5.346 | 3.200 ± 2.055 | 3.607 ± 3.359 | 1.4 | 0.253 |
| LAP | |||||||||||||
| Mean ± SD | 32.73 ± 25.24 A | 49.33 ± 25.38 B | 66.44 ± 46.54 B, C | 83.89 ± 65.45 C | 20.8 | < 0.001 | 53.90 ± 38.58 A | 73.13 ± 37.25 B | 116.92 ± 132.09 A, B | 90.86 ± 44.93 B | 107.34 ± 87.75 A, B | 20.8 | 0.001 |
Abbreviations: CVAI, Chinese Visceral Adiposity Index; VAI, Visceral Adiposity Index; LAP, lipid accumulation product.
a Similar superscript capital letter indicate that there is no significant difference between two levels in post HOC test.
The receiver of operating characteristic (ROC) curves for diagnosing S ≥ S1. Chinese Visceral Adiposity Index (CVAI): Area under the receiver operating characteristic curve (AUROC) = 0.800 (0.747 to 0.846), cut-point = 82.62, sensitivity (SN) = 91.86%, and specificity (SP) = 56.86%. LAP: Area under the receiver operating characteristic curve = 0.801 (0.749 to 0.847), cut-point = 41.76, SN = 73.42%, and SP = 74.51%. VAI: Area under the receiver operating characteristic curve = 0.715 (0.657 to 0.768), cut-point = 1.57, SN = 74.77%, and SP = 62.75%. WC: Area under the receiver operating characteristic curve: 0.811(0.760 to 0.856), cut-point = 90, SN = 81.08%, and SP = 68.63%. The AUROC of CVAI was not significantly different from LAP (P = 0.860) and WC (P = 0.264). However, VAI had significantly lower AUROC than CVAI (P = 0.047), WC (P = 0.031), and LAP (P < 0.001).
4.3. Chinese Visceral Adiposity Index, VAI, and Lipid Accumulation Product in Predicting, Diagnosis, and Grading of Liver Fibrosis
Comparison analysis revealed that CVAI values were higher in the significant fibrosis group compared to the non-significant fibrosis group (174.71 ± 44.41 vs. 120.24 ± 48.69, P < 0.001). The comparison results for VAI and LAP are presented in Table 1.
Univariate logistic regression showed that CVAI was significantly associated with significant fibrosis (OR = 1.028, CI 95%: 1.019-1.038, P < 0.001). After adjusting for age, gender, metabolic disorders, and other visceral markers, each unit increase in CVAI raised the odds of significant fibrosis by 2.9% (Adj. OR = 1.029, CI 95%: 1.011 - 1.047, P = 0.001). The logistic regression results for VAI and LAP are shown in Table 2.
According to ANOVA, the stages of liver fibrosis had a significant impact on CVAI [F (4, 267) = 17.239, P < 0.001]. Post hoc analysis indicated no significant difference in mean CVAI values between each two consecutive fibrosis stages. The mean and SD of VAI and LAP for each stage of liver fibrosis are presented in Table 3.
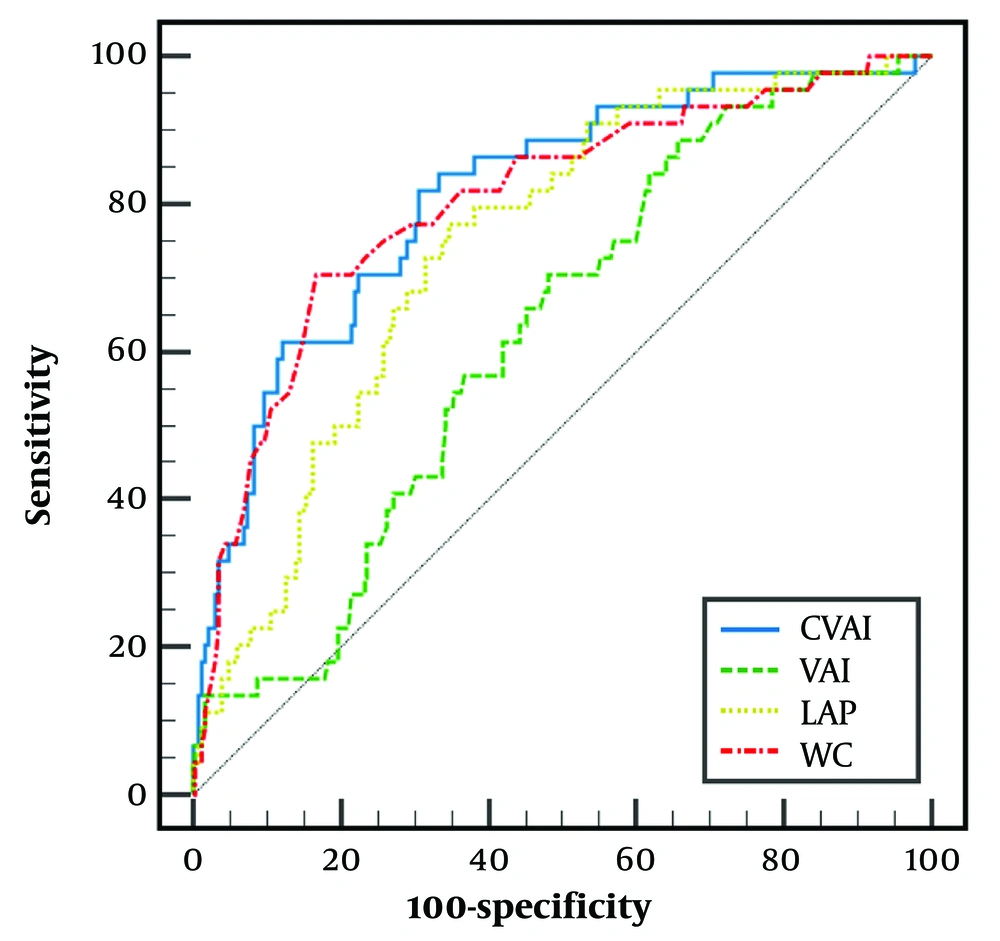
According to ROC analysis, for F ≥ F2, the AUROC of CVAI was 0.810 (95% CI: 0.759 to 0.855) with a cut-point of >142.42, sensitivity (SN) of 81.82%, and specificity (SP) of 69.30%. The AUROC, cut points, SN, and SP of other visceral fat markers are depicted in Figure 2.
The receiver of operating characteristic (ROC) curves for diagnosing F ≥ F2. Chinese Visceral Adiposity Index (CVAI): Area under the receiver operating characteristic curve (AUROC): 0.810 (0.759 to 0.855), cut-point = 142.42, sensitivity (SN) = 81.82, and specificity (SP) = 69.30. LAP: Area under the receiver operating characteristic curve = 0.745 (0.689 to 0.796), cut-point = 61.82, SN = 77.27, and SP = 65.50. VAI: Area under the receiver operating characteristic curve = 0.625 (0.565 to 0.683), cut-point = 1.53, SN = 88.64, and SP = 34.50. WC: Area under the receiver operating characteristic curve = 0.800 (0.748 to 0.846), cut-point = 104, SN = 70.45, and SP = 83.4. The AUROC of CVAI was not significantly different from WC (P = 0.579) and LAP (P = 0.086). However, VAI had significantly lower AUROC than CVAI (P < 0.001), WC (P = 0.001), and LAP (P < 0.001).
5. Discussion
The study aimed to evaluate the diagnostic value of CVAI for liver steatosis and fibrosis in the Iranian population. Our findings suggested that CVAI, in comparison with VAI or LAP, was a robust predictor of liver steatosis. A cross-sectional study on a Chinese population similarly found that all three visceral indices—CVAI, VAI, and LAP—were independently associated with an increased risk of developing MASLD. However, in prospective analysis, CVAI was shown to be a better predictor of MASLD over a three-year period compared to VAI, LAP, and WC (12). Current findings also indicated that CVAI has good accuracy in diagnosing hepatic steatosis. Nonetheless, CVAI showed no superiority over WC, which is a simple and cost-effective measure for evaluating visceral adiposity. Lipid accumulation product and CVAI had similar levels of accuracy in diagnosing liver steatosis, while VAI showed significantly weaker results than both LAP and CVAI.
Consistent with our findings, Chen et al. (12) observed that CVAI, LAP, and WC had good accuracy in diagnosing MASLD; however, the performance of VAI in differentiating MASLD patients from healthy individuals was only average. Our results also demonstrated that CVAI, unlike VAI and LAP, significantly predicts liver steatosis independently of obesity, dyslipidemia, and diabetes. An analysis of more than nine thousand Chinese subjects found that CVAI is an independent and strong predictor of MASLD in lean adults (26). Moreover, CVAI predicts the likelihood of developing MASLD in diabetic adults, independent of diabetes and other metabolic factors (34). Overall, CVAI may serve an important role in the screening and risk assessment of MASLD, regardless of age, gender, obesity status, and metabolic diseases.
CVAI was the only visceral adiposity marker that predicted the likelihood of significant fibrosis independently of obesity and other metabolic disorders. However, CVAI is not an efficient marker for differentiating between stages of liver fibrosis. Although Li et al. (35) found that CVAI, VAI, LAP, and WC are associated with higher MASLD activity and have a strong correlation with fibrosis, our findings suggested that only the association with CVAI remained significant after adjusting for confounders.
Accumulating evidence supports the role of VAT in MASLD pathogenesis through mechanisms such as insulin resistance, secretion of pro-inflammatory cytokines and leptin, and the release of high quantities of free fatty acids to the portal vein, which activates toll-like receptor 4 (21, 36-38). While VAI was an excellent surrogate marker for visceral fat volume and function in Caucasians, racial disparities in visceral fat distribution limit its applicability in Asian populations (18, 39). Consequently, CVAI was developed to estimate visceral fat volume and function specifically in the Chinese population. Chinese Visceral Adiposity Index has shown strong correlations with visceral obesity and the homeostatic model for insulin resistance (HOMA-IR). Additionally, CVAI has been shown to be superior to BMI and WC in assessing risk for metabolic disorders among normal-weight individuals with metabolic disorders and among obese individuals without metabolic diseases (22). These characteristics make CVAI a strong predictor and accurate diagnostic factor for hepatic steatosis and significant fibrosis.
To our knowledge, we are the first authors to investigate the value of CVAI in predicting and diagnosing liver steatosis and significant fibrosis in West Asia. Additionally, the use of elastography in this study enabled us to evaluate the utility of CVAI in grading liver steatosis and staging liver fibrosis, an area that has not been well studied previously. However, due to the cross-sectional nature of this study, a causal relationship between CVAI and liver steatosis or significant fibrosis cannot be established.
Although our results suggest a potential application of CVAI in MASLD screening, CVAI still requires improved sensitivity and specificity to serve as an ideal marker for MASLD. Other factors beyond visceral adiposity, such as insulin resistance (IR) and systemic inflammation (40), significantly influence the development and progression of MASLD. Including these factors in the CVAI formula may enhance its predictive value and diagnostic power for liver steatosis and fibrosis, as well as that of similar markers.
5.1. Conclusions
In conclusion, our study highlights the superior predictive value of CVAI in diagnosing liver steatosis compared to other visceral adiposity markers, such as VAI and LAP. Notably, the predictive power of CVAI was independent of factors like obesity, dyslipidemia, and diabetes. While CVAI showed good accuracy in detecting hepatic steatosis, it did not surpass the simplicity and cost-effectiveness of WC as a measure of visceral fat. Additionally, both CVAI and LAP demonstrated comparable accuracy, while VAI showed weaker performance. Furthermore, CVAI was able to predict the likelihood of significant fibrosis independently of metabolic disorders, although it was not effective in distinguishing between different stages of liver fibrosis. Therefore, we propose that CVAI is a valuable tool for screening and risk assessment in MASLD, underscoring the important role of visceral fat in liver health.