1. Background
Hepatitis B is a common and frequently occurring disease in clinical practice. There are 900,000 to 1,000,000 new cases of hepatitis B in China every year, and both the number of new cases and the incidence rate are on the rise (1). Currently, antiviral medication is the dominant clinical treatment for hepatitis B, effectively delaying disease progression (2). Nevertheless, hepatitis B affects liver microcirculation and suppresses normal physiological function, potentially inducing decompensated cirrhosis in the presence of various internal and external factors (3, 4). Decompensated hepatitis B cirrhosis (HBC) is characterized by symptoms and signs caused by hepatic insufficiency and portal hypertension, accompanied by various complications, making treatment more difficult and greatly increasing the risk of poor prognosis (5, 6). The high incidence rate of cirrhosis-related complications in patients with decompensated HBC not only brings significant economic and psychological burdens but also markedly increases the risk of death (7, 8). However, recompensation can occur in some patients with decompensated cirrhosis after actively receiving etiological and symptomatic treatment as early as possible, thus transitioning to compensated cirrhosis (9, 10). Therefore, it is of great significance to identify the influencing factors for poor prognosis in patients with decompensated HBC as early as possible and to screen high-risk groups to ameliorate prognosis. Although several studies have attempted to identify prognostic factors and develop predictive models for these patients, limitations such as small sample sizes, retrospective designs, and inadequate adjustment for confounding factors exist (11, 12).
2. Objectives
In this context, the influencing factors for poor prognosis of decompensated HBC subjects were analyzed in this study, and a risk prediction model was established based on multivariate logistic regression analysis, aiming to improve the prognosis of patients through early prevention.
3. Methods
3.1. General Data
In this prospective study, 120 subjects were selected from patients with decompensated HBC who were hospitalized for treatment between January 2022 and January 2023. This study was reviewed and approved by the medical ethics committee of our hospital (approval No. 2021001204).
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were determined as follows: (1) Patients with a history of hepatitis B and clinically diagnosed with decompensated HBC; (2) those who signed informed consent forms; (3) those with clear consciousness, a normal mental state, and stable vital signs, allowing them to cooperate in the study; (4) those with a history of hepatitis B; (5) those with an expected survival period greater than 6 months; and (6) those with complete clinical and imaging data.
Exclusion criteria included: (1) Patients with uncontrolled infectious or communicable diseases; (2) those with severe anemia or poor general conditions; (3) those with decompensated cirrhosis due to alcoholic liver disease, cholestatic liver disease, or autoimmune liver disease; (4) those with a history of splenectomy or portal shunt; (5) those with a medication history of glucocorticoids or immunosuppressants; or (6) those with coagulation disorders or bleeding tendencies.
3.3. Prognostic Evaluation
The patients were followed up for one year through telephone or in-person visits at regular intervals. With death during follow-up as the prognostic endpoint, the patients were assigned to a good prognosis (survival) group (n = 90) and a poor prognosis (death) group (n = 30).
3.4. Collection of Baseline Data
A questionnaire was designed to collect demographic data from patients, including age, sex, smoking history, underlying disease, drinking history, family history, international normalized ratio (INR) (prothrombin time/international sensitivity index), and hypoproteinemia (defined as plasma total protein < 60 g/L and albumin < 30 g/L).
Relevant laboratory indicators were measured at the time of enrollment. Specifically, 3 mL of fasting venous blood was drawn in the morning on the first day after admission and centrifuged to obtain the upper serum. Liver function indicators—including alkaline phosphatase, gamma-glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, and total bilirubin—and kidney function indicators—including serum creatinine, blood urea nitrogen, cystatin C, and glomerular filtration rate—were measured using the 7060 automatic biochemical analyzer (Hitachi, Japan).
The inflammatory marker high-sensitivity C-reactive protein was examined using an enzyme-linked immunosorbent assay (ELISA) with a kit purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (China). Routine blood indicators—including hemoglobin count, platelet count, and white blood cell count—were measured using the Countstar cell analyzer (Beijing Taize Jiaye Technology Development Co., Ltd., China).
Blood lipid indicators, including triglycerides and total cholesterol (TC), were detected using the AU5821 automatic biochemical analyzer (Beckman Coulter, USA). Cell-free deoxyribonucleic acid (cfDNA) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were assessed using polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay, respectively (kits: Genzyme, USA).
3.5. Statistical Analysis
SPSS version 23.0 software was utilized for statistical analysis. Measurement data were described as mean ± standard deviation
4. Results
4.1. Prognosis
There were 30 cases (25%) in the poor prognosis group and 90 cases (75%) in the good prognosis group.
4.2. Baseline Data of Two Groups
No significant differences were found between the two groups in terms of sex, age, underlying diseases, smoking history, drinking history, family history, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total bilirubin, serum creatinine, blood urea nitrogen, cystatin C, glomerular filtration rate, high-sensitivity C-reactive protein, hemoglobin count, platelet count, white blood cell count, and triglyceride levels (P > 0.05). However, the proportion of patients with hypoproteinemia, as well as the serum levels of INR, TC, cfDNA, and GM-CSF, were significantly higher in the poor prognosis group compared to the good prognosis group (P < 0.05) (Table 1).
| Factors | Poor Prognosis Group (n = 30) | Good Prognosis Group (n = 90) | χ2/t Value | P-Value |
|---|---|---|---|---|
| Gender | 0.011 | 0.916 | ||
| Male (n = 65) | 16 (53.33) | 49 (54.44) | ||
| Female (n = 55) | 14 (46.67) | 41 (45.55) | ||
| Underlying disease | 0.019 | 0.890 | ||
| Yes (n = 21) | 5 (16.67) | 16 (17.78) | ||
| No (n = 99) | 25 (83.33) | 74 (82.22) | ||
| Family history | 0.626 | 0.429 | ||
| Yes (n = 5) | 0 (0.00) | 5 (5.56) | ||
| No (n = 115) | 30 (100.00) | 85 (94.44) | ||
| Smoking history | 0.029 | 0.865 | ||
| Yes (n = 13) | 3 (10.00) | 10 (11.11) | ||
| No (n = 107) | 27 (90.00) | 80 (88.89) | ||
| Drinking history | 0.021 | 0.885 | ||
| Yes (n = 19) | 5 (16.67) | 14 (15.56) | ||
| No (n = 101) | 25 (83.33) | 76 (84.44) | ||
| Hyperproteinemia | 6.698 | 0.010 | ||
| Yes (n = 81) | 26 (86.67) | 55 (61.11) | ||
| No (n = 39) | 4 (13.33) | 35 (38.89) | ||
| Age (y) | 55.86 ± 5.25 | 55.65 ± 5.34 | 0.187 | 0.852 |
| Aspartate aminotransferase (U/L) | 55.55 ± 10.25 | 53.24 ± 10.05 | 1.085 | 0.280 |
| Alanine aminotransferase (U/L) | 43.52 ± 6.25 | 45.24 ± 6.52 | 1.264 | 0.209 |
| Alkaline phosphatase (U/L) | 130.24 ± 15.65 | 133.54 ± 16.24 | 0.972 | 0.333 |
| Gamma-glutamyl transpeptidase (U/L) | 72.54 ± 7.58 | 71.24 ± 8.40 | 0.751 | 0.454 |
| Total bilirubin (μmol/L) | 50.24 ± 6.24 | 48.54 ± 7.25 | 1.150 | 0.253 |
| Serum creatinine (μg/L) | 75.21 ± 8.21 | 72.25 ± 7.26 | 1.871 | 0.064 |
| Blood urea nitrogen (mmol/L) | 7.50 ± 0.52 | 7.36 ± 0.32 | 1.752 | 0.082 |
| Cystatin C (mg/L) | 1.49 ± 0.23 | 1.47 ± 0.21 | 0.441 | 0.660 |
| Glomerular filtration rate (mL/min) | 155.72 ± 18.85 | 153.45 ± 18.32 | 0.584 | 0.561 |
| High-sensitivity C-reactive protein (mg/L) | 10.33 ± 0.99 | 10.24 ± 0.88 | 0.470 | 0.639 |
| Hemoglobin count (g/L) | 110.52 ± 15.25 | 112.24 ± 16.24 | 0.451 | 0.653 |
| Platelet count (× 109 L) | 55.35 ± 5.24 | 54.24 ± 5.02 | 1.038 | 0.302 |
| White blood cell count (× 109 L) | 6.02 ± 0.15 | 5.99 ± 0.16 | 0.903 | 0.368 |
| Triglyceride (mmol/L) | 1.15 ± 0.30 | 1.20 ± 0.25 | 0.901 | 0.369 |
| Total cholesterol (mmol/L) | 2.19 ± 0.22 | 3.26 ± 0.33 | 16.552 | < 0.001 |
| Cell free deoxyribonucleic acid (μg/L) | 3571.25 ± 416.27 | 3016.52 ± 342.25 | 7.272 | < 0.001 |
| Granulocyte-macrophage colony stimulating factor (ng/mL) | 35.15 ± 3.02 | 25.12 ± 2.92 | 16.156 | < 0.001 |
| International normalized ratio | 1.81 ± 0.40 | 1.50 ± 0.32 | 4.307 | < 0.001 |
a Values are expressed as No. (%) or mean ± SD.
4.3. Results of Multivariate Logistic Regression Analysis
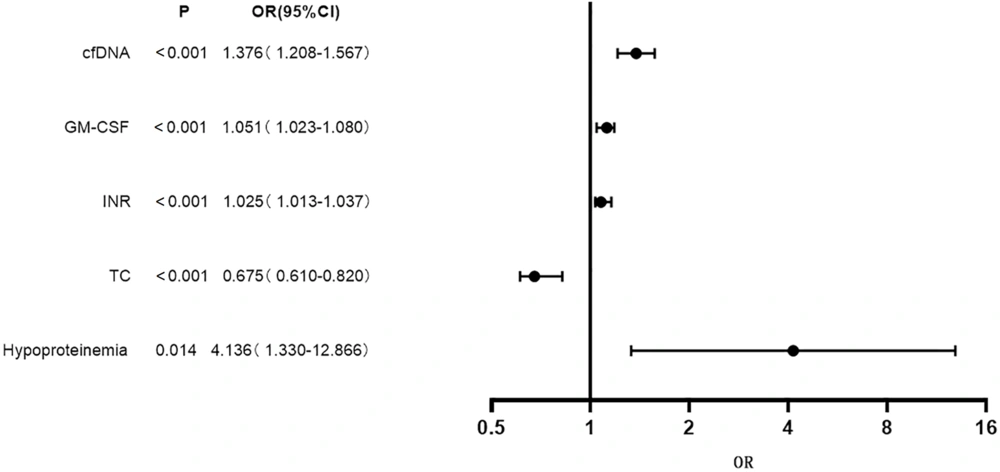
Using the indicators associated with poor prognosis in patients with decompensated HBC (TC, cfDNA, GM-CSF, INR, and hypoproteinemia) as independent variables (with assigned values shown in Table 2), and patient prognosis as the dependent variable (1 = Poor prognosis, 0 = Good prognosis), multivariate logistic regression analysis was performed. The results indicated that hypoproteinemia, cfDNA, GM-CSF, and INR were risk factors for poor prognosis in patients with decompensated HBC [odds ratio (OR) > 1, P < 0.05], while TC was identified as a protective factor (OR < 1, P < 0.05) (Table 3 and Figure 1).
| Variables | Variable Category | Description | Assignment |
|---|---|---|---|
| Total cholesterol | Continuous variable | X1 | - |
| Cell free deoxyribonucleic acid | Continuous variable | X2 | - |
| Granulocyte-macrophage colony stimulating factor | Continuous variable | X3 | - |
| International normalized ratio | Continuous variable | X4 | - |
| Hypoproteinemia | Categorical variable | X5 | 1 = Yes, 0 = No |
| Prognosis | Categorical variable | Y | 1 = Poor prognosis, 0 = Good prognosis |
| Indicator | β | Standard Error | Wald | P | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| cfDNA | 0.319 | 0.066 | 23.075 | < 0.001 | 1.376 | 1.208 - 1.567 |
| Granulocyte-macrophage colony stimulating factor | 0.050 | 0.014 | 12.867 | < 0.001 | 1.051 | 1.023 - 1.080 |
| International normalized ratio | 0.024 | 0.006 | 15.893 | < 0.001 | 1.025 | 1.013 - 1.037 |
| Total cholesterol | -0.397 | 0.109 | 25.214 | < 0.001 | 0.675 | 0.610 - 0.820 |
| Hypoproteinemia | 1.420 | 0.579 | 6.014 | 0.014 | 4.136 | 1.330 - 12.866 |
| Constant | -12.544 | 3.201 | 15.352 | 0.000 | 0.000 | - |
Abbreviation: cfDNA, cell-free deoxyribonucleic acid.
4.4. Establishment and Evaluation of Prediction Model
A regression equation was established as follows:
Logit (P) = -12.544 + 1.376 × cfDNA + 1.051 × GM-CSF + 1.025 × INR + 0.675 × TC + 4.136 × hypoproteinemia.
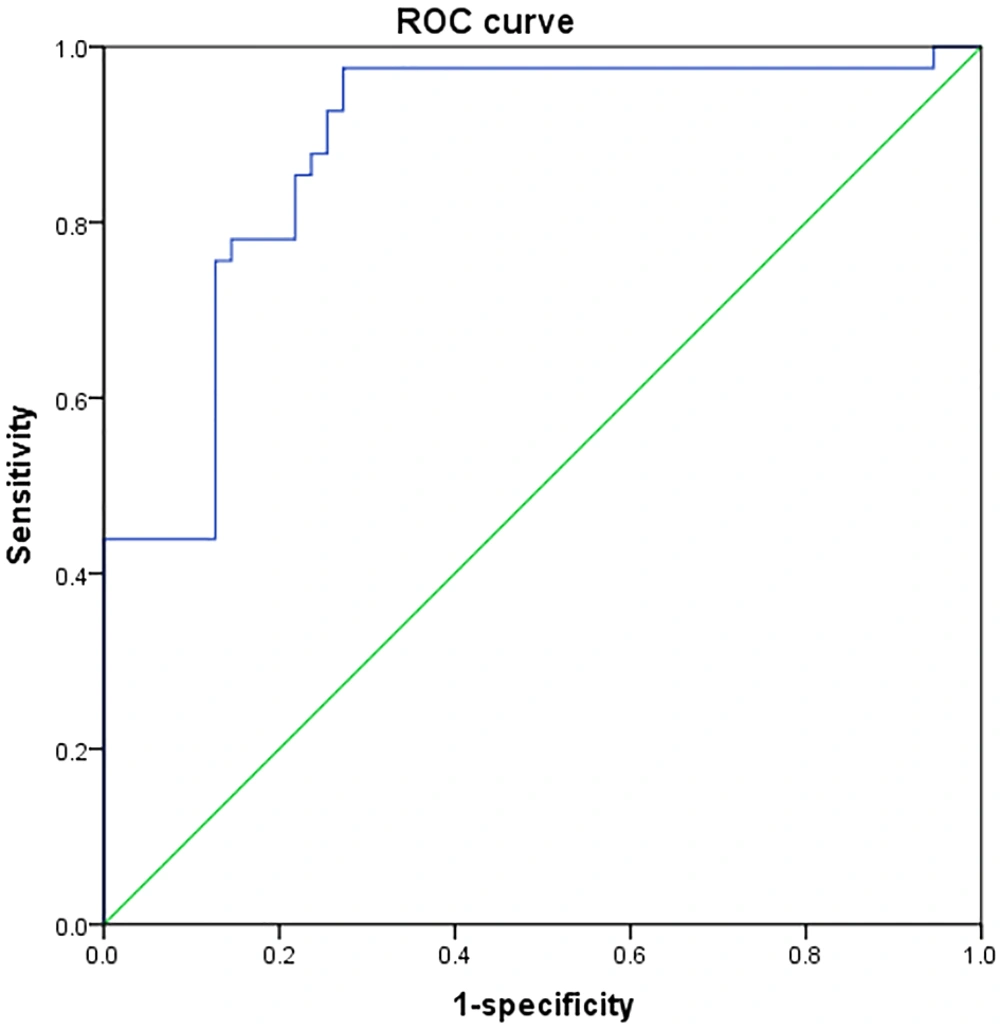
The area under the ROC curve for the prediction model was 0.920 [95% confidence interval: 0.855 - 0.980, P < 0.001], indicating that the model was both reliable and stable (Figure 2).
5. Discussion
In this study, we intended to explore the prognostic factors in patients with decompensated HBC and to establish a risk prediction model. The results of logistic regression analysis revealed that hypoproteinemia, cfDNA, GM-CSF, and INR were risk factors for poor prognosis in patients with decompensated HBC, while TC served as a protective factor. The reasons are as follows:
First, liver damage caused by decompensated HBC weakens the liver’s capacity for protein and plasma protein synthesis. With the continuous decline in plasma albumin, the effective osmotic pressure also decreases, leading to excessive water retention in tissues. This results in complications such as pleural effusion and ascites, significantly increasing the difficulty of treatment and the risk of poor prognosis. Additionally, these patients often experience impaired blood circulation and reduced oxygen-carrying capacity, contributing to insufficient tissue oxygen supply and further elevating the risk of unfavorable outcomes (13, 14). Therefore, it is essential to assess hypoproteinemia and implement appropriate corrective interventions to improve nutritional status and thereby ameliorate prognosis.
Second, cfDNA is typically released by lymphocytes, other nucleated cells, damaged tissue cells, and inflammatory cells. It is an effective biomarker for apoptosis and/or tissue injury, and its concentration in circulation reliably reflects the severity of tissue damage (15, 16). Elevated cfDNA levels are indicative of more extensive hepatic tissue damage in patients with decompensated HBC, leading to an inevitably poor prognosis despite active treatment.
Third, GM-CSF promotes the proliferation and activation of macrophages and stimulates the release of T-cell activation-related cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Elevated TNF-α levels exacerbate the hepatic inflammatory response and enhance the expression of human leukocyte antigen class I (HLA-I) and intercellular adhesion molecules. HLA-I is associated with the recognition of target antigens by cytotoxic T lymphocytes, while intercellular adhesion molecules facilitate the adhesion of these lymphocytes to hepatocytes. This process, driven by NF-κB activation, promotes hepatocyte fibrosis and liver injury, thus increasing the likelihood of a poor prognosis (17, 18)
Interleukin-6, through activation of the JAK/STAT3 signaling pathway, contributes to the proliferation and activation of hepatocytes and astrocytes, thereby worsening liver fibrosis and portal hypertension. Additionally, IL-6 regulates T-cell polarization by promoting the differentiation and activation of T helper 17 (Th17) cells, which compromises the liver’s immune defense mechanisms. Consequently, disease progression continues, resulting in a poor prognosis (19, 20).
Fourth, an increased INR reflects a more pronounced decline in liver synthetic and reserve capacity, more significant hypersplenism, and a more severe overall condition, thereby greatly increasing the risk of poor prognosis (21).
Fifth, TC levels remain in dynamic balance in individuals with normal liver function. However, in patients with decompensated HBC, whose liver function is notably impaired, serum TC levels decline due to a reduction in active hepatocytes and inhibited synthesis of phospholipid-cholesterol acyltransferase and apolipoprotein A (22, 23). Thus, a lower serum TC level indicates more substantial liver damage, subsequently elevating the risk of a poor prognosis.
Taken together, it is essential to closely monitor cfDNA, GM-CSF, INR, and TC levels in patients and implement effective interventions to maintain these indicators within the normal range, thereby reducing the risk of poor outcomes.
Finally, a regression equation was constructed as follows:
Logit (P) = -12.544 + 1.376 × cfDNA + 1.051 × GM-CSF + 1.025 × INR + 0.675 × TC + 4.136 × hypoproteinemia.
This comprehensive prediction model, developed using five independent risk factors, effectively compensates for the low sensitivity and specificity of univariate prediction. It aids in the rapid identification of high-risk patients with poor prognoses due to decompensated HBC, thereby supporting clinical decision-making and improving outcomes.
However, this study has several limitations. The patient population was derived from a limited geographic and clinical range, which may introduce selection bias. In addition, some patients presented with other complications, making it challenging to isolate the effects of individual variables. Moreover, the follow-up duration was relatively short. Therefore, future validation of this risk prediction model will require multicenter, prospective studies with larger sample sizes and extended follow-up periods.
In conclusion, cfDNA, GM-CSF, INR, and hypoproteinemia are independent risk factors for poor prognosis in patients with decompensated HBC. The prediction model developed in this study demonstrates strong predictive performance and offers a valuable reference for future clinical assessment and intervention strategies.