1. Introduction
Hepatocellular carcinoma (HCC) is a growing global health concern, with over 80% of patients being inoperable (1). The majority of patients undergo local, regional, or systemic palliative therapies (2). Local treatment, such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and radiation therapy, combined with immune checkpoint inhibitors (ICIs) against HCC, can have a synergistic effect (3-5). Selective internal radiation therapy (SIRT) has emerged as an effective locoregional treatment for unresectable HCC (6, 7). Selective internal radiation therapy involves the delivery of yttrium-90 microspheres via hepatic arterial infusion, selectively targeting tumor tissue while sparing healthy liver parenchyma. This approach has shown promising results in terms of tumor response and overall survival, particularly in patients with advanced HCC (8, 9). The abscopal effect, first noted by Mole in 1953, describes tumor regression outside the irradiated region (10). Dagoglu et al.’s review reported that radiation therapy combined with ICIs can lead to the abscopal effect (11). However, little is known about the association between the induction of the abscopal effect and SIRT against HCC. Here, we present a patient with multifocal HCC who received SIRT and RFA followed by ICI therapy and was found to have remarkable regression in a lesion outside the area targeted by SIRT and RFA.
2. Case Presentation
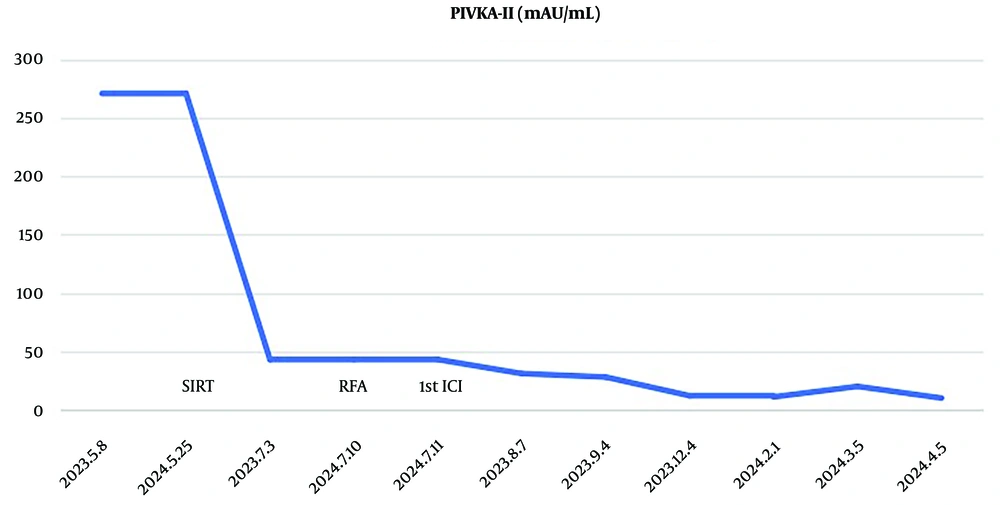
On 7 May 2023, a 76-year-old man was admitted with a history of stroke lasting 1 week and a history of chronic hepatitis B for more than 20 years. His MRI results showed a mass measuring 7.8 × 7.4 cm in segment 8 of the right hepatic lobe, a nodule measuring 2.9 × 2.7 cm in segment 7 of the right hepatic lobe, and other nodules in the left hepatic lobe. His MR examination indicated that the patient had cirrhosis. Serum alpha-fetoprotein (AFP) and prothrombin induced by vitamin K absence/antagonist-II (PIVKA-II) levels were 2.36 ng/mL (the normal reference range for AFP levels is between 0 ng/mL and 7 ng/mL) and 271 mAU/mL (the normal reference range for PIVKA-II levels is < 40 mAU/mL), respectively. Therefore, he was diagnosed with China Liver Cancer (CNLC) stage IIb HCC (12) along with chronic hepatitis B and cirrhosis.
According to the CNLC staging system, treatment options for stage IIb HCC include surgery, TACE, and systemic anti-tumor therapy. However, the patient's multifocal tumors in both the left and right lobes of the liver make surgical resection unsuitable. Additionally, given the patient’s acute stroke and weakened condition, the nausea and vomiting commonly associated with TACE could be life-threatening, rendering this option inappropriate. At present, there is insufficient evidence to support the use of tyrosine kinase inhibitors in similar special populations. For inoperable HCC patients who do not receive active treatment, the median survival time is only 4.2 months (13). However, SIRT, which selectively injects radioactive microspheres into tumor-supplying arteries via arterial catheters without the use of chemotherapy drugs, offers a safer approach to tumor control (14). Therefore, this method may provide survival benefits for the patient.
After consensus from the multidisciplinary liver tumor board, SIRT was recommended to the patient for palliative therapy. Radiofrequency ablation and an anti-PD-1 antibody were recommended as subsequent combination therapy methods.
2.1. Treatment Strategy
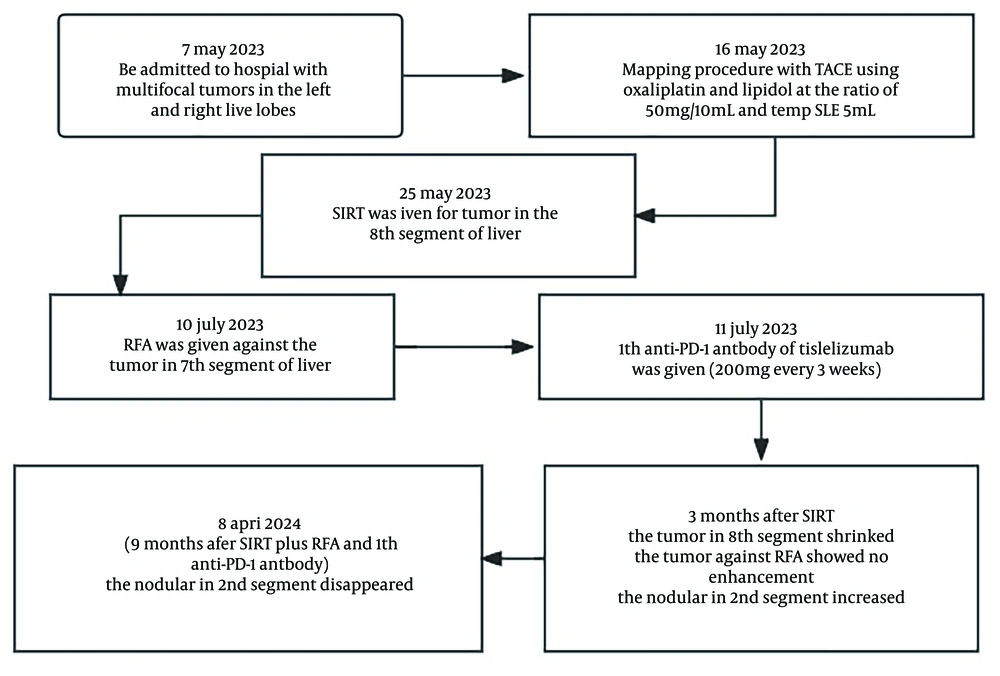
On 16 May 2023, a mapping procedure was carried out before SIRT. The angiography revealed an aberrant vessel originating from the right diaphragmatic artery that was involved in the blood supply to some tumors. A microcatheter was used to enter the artery, and chemoembolization was performed by mixing oxaliplatin with lipiodol in a ratio of 50 mg/10 mL, using a thermosensitive liquid embolic agent (TempSLE) (5 mL) and lipiodol lotion (5 mL). After embolization, the blood flow to the tumor blood vessels was obstructed, and the staining of surrounding tumors disappeared. The main artery supplying blood to the tumor in the 8th segment of the right lobe of the liver came from the right hepatic artery. The partition model was employed to assess the prescription activity, which was 0.9 GBq at a 110 Gy tumor dose. The patient received SIRT on 25 May. In the right hepatic artery, a 2.6 Fr microcatheter was superselectively placed, and under fluoroscopic guidance, 90Y resin microspheres (0.9 GBq; SIR-Spheres®, Sirtex Medical) were administered. The dose delivery was verified 1 hour after injection via a bremsstrahlung scan by liver SPECT/CT. Mild anorexia was observed on the first day after SIRT, which disappeared on the second day.
One and a half months post-SIRT, his MRI results showed that the tumor in segment 8 had slight regression, while the remaining intrahepatic nodules showed no significant changes. On 10 July 2023, RFA was carried out against the nodule measuring 2.9 × 2.7 cm in segment 7 of the right hepatic lobe. On the second day post-RFA, the patient was treated with an anti-PD-1 antibody, tislelizumab (200 mg every 3 weeks).
3. Results
Three months post-SIRT, the tumor in segment 8 decreased to 6.8 × 6.5 cm and then further reduced to 4.3 × 3.6 cm on 8 April 2024. Two months after RFA, the nodule treated with RFA in segment 7 showed no enhancement and then further regression on 8 April 2024. Three months after SIRT, the nodular HCC in segment 2, which was outside the regions treated with SIRT or RFA, increased. However, nine months after the treatment of SIRT and RFA combined with an anti-PD-1 antibody, the nodular HCC in segment 2 remarkably disappeared while the tumor in segment 8 showed no enhancement (Figure 1). Serum PIVKA-II dropped to normal levels two months post-SIRT (Figure 2). Currently, the patient is undergoing treatment with tislelizumab (200 mg every 3 weeks). Figure 3 shows the flowchart of the procedure. The patient was followed up until 15 August 2024, when PD-1 therapy was discontinued, and a liver MRI examination showed no tumor recurrence. During the recovery period following rehabilitation treatment for the stroke, the patient was able to walk freely and take care of herself.
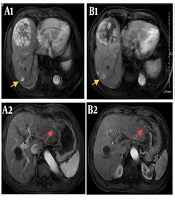
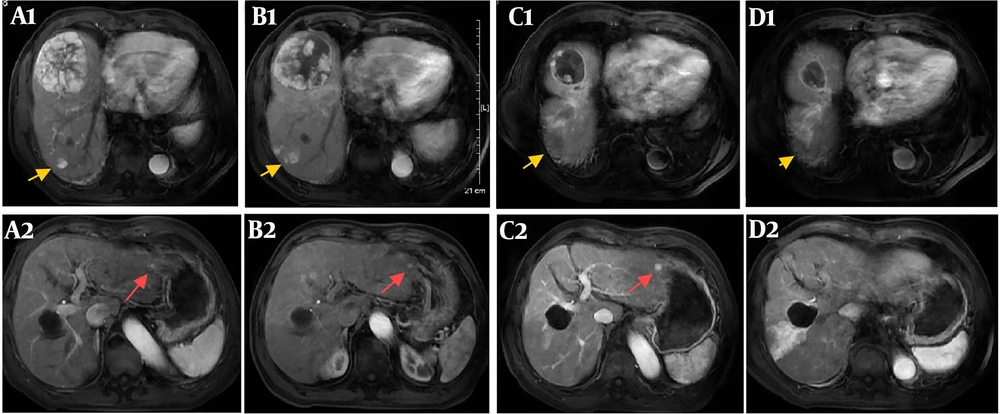
(A1 - A2): Enhanced magnetic resonance imaging on 6 May 2023 before selective internal radiation therapy (SIRT) showed the large hepatocellular carcinoma (HCC) (7.8 × 7.4 cm) in segment 8 of the right hepatic lobe, a nodular HCC (2.9 × 2.7 cm) in segment 7 of the right hepatic lobe (yellow arrow), and a nodular HCC < 1 cm in segment 2 of the left hepatic lobe (red arrow). (B1 - B2): Enhanced magnetic resonance imaging on 3 July 2023 after SIRT showed that the large HCC in segment 8 regressed while the other nodules remained similar to before. (C1 - C2): Enhanced magnetic resonance imaging on 1 September 2023 after radiofrequency ablation (RFA) showed that the large HCC in segment 8 further regressed, the nodular HCC in segment 7 treated by RFA showed no enhancement, while the nodular HCC in segment 2 increased. (D1 - D2): Enhanced magnetic resonance imaging on 8 April 2024 showed that the large HCC in segment 8 further regressed (4.3 × 3.6 cm), the nodular HCC in segment 7 treated by RFA showed no enhancement, while the nodular HCC in segment 2 disappeared.
4. Discussion
In recent years, the progress of ICIs in treating unresectable HCC has been significant. Compared with sorafenib, both atezolizumab plus bevacizumab and tremelimumab plus durvalumab regimens have achieved significant survival improvements (15, 16). Accumulating evidence also suggests that local treatments, such as TACE, RFA, or radiation therapy, combined with ICIs can effectively regulate the immune microenvironment of HCC, resulting in synergistic effects (3-5, 17, 18).
In the field of radiation oncology, the term abscopal effect describes a systemic anti-tumor immune response characterized by tumor regression outside of the irradiated region (11). After irradiation of tumor cells, tumor antigen release is taken up by antigen-presenting cells, such as dendritic cells, which then migrate to lymph nodes and activate CD8+ T-cells that gain the ability to differentiate into cytotoxic T lymphocytes capable of migrating to tumor sites and killing tumor cells (19). Although the phenomenon of the abscopal effect has attracted attention in the treatment of HCC, most studies on this effect have been conducted in the context of external beam radiotherapy (EBRT).
Abscopal effects after EBRT have mostly been reported in sporadic cases, and the overall proportion is low (20, 21). The proportion of complete responses in HCC treated with single ICIs is also very low, approximately 2.2% (22). Demaria et al. (23) first provided evidence that an RT-induced abscopal effect is an immune-mediated response. However, abscopal effects are increasingly being reported when radiotherapy is administered concomitantly with ICIs.
From the perspective of radiobiology, the long-term delivery of SIRT is very different from that of EBRT, and it remains uncertain how SIRT can also produce the abscopal effect (24). In a retrospective study, Powerski et al. found that the occurrence of the abscopal effect following SIRT was only 1% in 96 liver malignancies without cirrhosis (25).
There have been past cases where local treatment with TACE combined with TKI plus ICI has resulted in tumors disappearing far from the regions treated with TACE, suggesting that TACE combined with immunotherapy can promote the occurrence of the abscopal effect (18). The present patient received multiple local treatments, including SIRT, TACE, and RFA, followed by ICI therapy. Selective internal radiation therapy was performed on the tumor in segment 8 of the right hepatic lobe. Radiofrequency ablation was conducted on the tumor in segment 7 of the right hepatic lobe. However, an anti-tumor effect was detected in the left hepatic lobe, far from the site targeted by local treatments, during the subsequent course of ICI therapy. In this case, enhanced magnetic resonance imaging on 8 April 2024 showed that the large HCC in segment 8 further regressed to 4.3 × 3.6 cm without enhancement; the nodular HCC in segment 7, treated by RFA, showed no enhancement, while the nodular HCC in segment 2 disappeared. Therefore, the patient was classified as having a complete response according to the mRECIST criteria. Both the tumor in segment 8 treated with SIRT and the tumor in segment 2, outside the regions treated with SIRT or RFA, achieved complete remission simultaneously.
Previous studies have shown that both SIRT and RFA can effectively present tumor antigens and induce T-cell extravasation (4, 26). The T-cell immune response induced by RFA leads to detectable anti-tumor reactions (27). PD-L1, PD-1, and CTLA-4 help inhibit the anti-tumor activity induced by thermal ablation (27). The combination of ICIs with SIRT or RFA can increase the incidence of abscopal effects (28).
We conclude that the combination of SIRT, RFA, and ICIs resulted in an enhanced anti-tumor immune response, primarily manifested as an abscopal effect. There are several limitations in this report: (1) the patient received multiple local treatment methods, including SIRT and RFA combined with ICI therapy, and it is currently uncertain which method plays a decisive role in the disappearance of the tumor far from the local treatment area; (2) the effect of simple immunotherapy cannot be ruled out.
Therefore, it is necessary to further accumulate cases that demonstrate anti-tumor effects when treated with SIRT plus RFA followed by ICI in order to confirm the abscopal effect induced by multiple local treatments combined with ICI in HCC patients.
4.1. Conclusions
In summary, our findings suggest that multiple local treatment methods combined with ICI therapy can benefit patients with multifocal HCC. Further clinical trials are required to validate these results for future application.