1. Background
Infection with hepatitis C virus (HCV) could end up with both acute and chronic hepatitis. Although acute infection rarely causes hepatic failure but in 75 – 85 percent of cases it would be developed to chronic infection (1). Chronic HCV infection is usually slowly progressive and in most of patients may not result in clinically apparent liver disease; however, cirrhosis would occur in approximately 20 – 30 percent of chronically infected patients over 20 – 30 years. The prevalence of hepatitis C varies between different regions but the World Health Organization (WHO) has estimated the number of chronically infected hepatitis C patents around 150 million globally (2) which is consistent with other studies (3). Its prevalence in Iran is less than 1% in general population (4). The prevalence of chronic hepatitis C infection in patients with beta thalassemia major is 18%, particularly because of blood transfusion before HCV serological testing (1, 5).
Hepatitis C imposes considerable burden to the global healthcare system in both acute and chronic forms. Global disability adjusted life years (DALYs) per 100,000 population in 2010 in comparison with 1990 has been estimated to have 44.4% growth in acute hepatitis C and also 21.3% and 1.9% growth in liver cancer and cirrhosis of the liver secondary to hepatitis C, respectively (6). This disease also reduces the quality of life in patients (7).
Treatment with antiviral agents could eradicate the virus in the serum and hepatocytes. There are several regimens to treat chronic hepatitis C. Most recently, pegylated interferon (PEG-IFN) as a new generation IFN has shown improvement in response rate (8). Pegylation of IFN also extends the half-life of medicine from a few hours to several days, therefore the injection intervals is decreased from 3 times a week to only once a week (9) which could increase patients' compliance. Peginterferon alfa-2a (PEG IFN alfa-2a) and peginterferon alfa-2b are the two types of PEG-IFN which are both approved by the US Food and Drug Administration (FDA) in treatment of hepatitis C which are different in some structural and pharmacokinetic characteristics (10) as well as effectiveness properties (11-15). Combination (or dual) therapy with PEG-IFN plus Ribavirin as an antiviral medicine has shown a promising effectiveness and also acceptable compliance in many studies (16), and also in Iranian patients (17). In spite of availability and utilization of these medical treatments in Iran, there is not any published economic evaluation to compare these alternatives from Iranian perspective.
2. Objectives
In this study, we aimed to analyze the cost effectiveness of treatment with PEG IFN alfa-2a plus Ribavirin in comparison with PEG IFN alfa-2a alone in hepatitis C infected thalassemia patients in Iran.
3. Patients and Methods
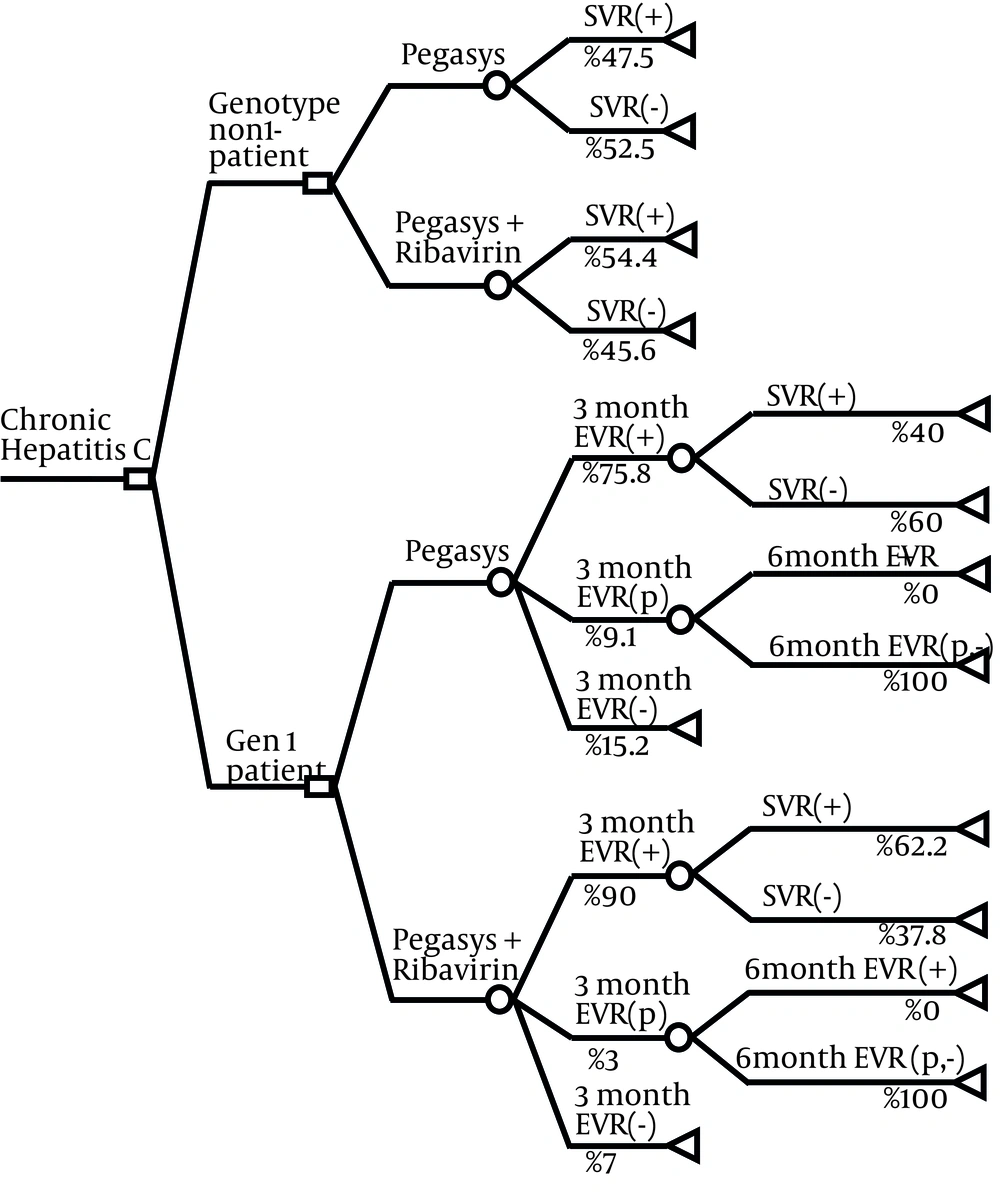
We developed a decision analytic model to assess the cost and effectiveness of two available treatment strategies which are:
1. Weekly subcutaneous injection of PEG IFN alfa-2a (Pegasys).
2. Weekly subcutaneous injection of PEG IFN alfa-2a plus daily use of Ribavirin oral tablets (see Figure 1).
The society was used as perspective of study. To develop decision tree model and all analysis, we used Tree Age Pro 2011 and Microsoft Excel 2007.
3.1. Patients and Treatment Strategies
This model was designed and run for a hypothetical cohort of 100 thalassemia major patients with diagnosis of chronic hepatitis C infection. Combination treatment strategy was defined as receiving 180 microgram (μg) of PEG IFN alfa-2a, subcutaneously once a week in combination with oral Ribavirin (600 – 800 mg per day based on patients’ hemoglobin level). In this strategy, patient with genotype-1 infection would be treated for 48 weeks and the other genotypes for 24 weeks. Monotherapy strategy was defined as receiving 180 μg of PEG IFN alfa-2a for 48 weeks.
A baseline viral load would be obtained from patients before beginning the treatment. In genotype-1 patients, the viral load test would be repeated after three months of therapy and compared with the baseline. In patients with complete Early Virological Response (EVR), indicating no detectable HCV RNA, or partial EVR, indicating at least a two log reduction from baseline viral load, the treatment would be continued because there is a high probability of success at the end of treatment course. In other patients who do not achieve complete or partial EVR, treatment success is highly unlikely, therefore treatment would be stopped because of potential adverse effects and costs and very low probability of success at the end. For partial responder patients, this procedure would be repeated again in sixth month and for patients without complete EVR at sixth month, treatment would be discontinued. Checking EVR would be less useful in genotypes non-1 due to the shorter treatment course and higher response rates. So treatment of genotype-1 patients would be discontinued at third month for patients in the category of no response, and at sixth month for partial responders who do not achieve complete response. More details about the treatment strategies are represented elsewhere (18).
3.2. Data Sources for Decision Tree Model
3.2.1. Effectiveness and Outcomes of Interest
All effectiveness and safety data including different probabilities were extracted from a domestic clinical trial, conducted in Baqiyatallah University of Medical Sciences in 2007 which was about combination treatment of HCV in 180 thalassemia major patients (18). We did not use any international evidence, mostly because of less similarity with real situation of Iranian setting of treatment. The time period of this model was until the end of follow up course, which was 72 weeks in most of the patients so the longer time side effects were not taken into account. In this study the patients were categorized regarding three parameters: HCV genotype (genotype-1 and non-1), previous treatment outcome (naïve, resistant to previous monotherapy and resistant to combination therapy), and the basic viral load (low viral load (< 600,000 IU/mL) and high viral load (> 600,000IU/mL).
Final outcome was divided into sustain responder (SVR+) and sustain nonresponder (SVR-). Sustain Virological Response (SVR) is defined as no detectable viral level (the absence of hepatitis C RNA) after six months from completion of therapy (success) (19), and Sustain nonresponder (failure) consist of five situations including response then relapse (detected HCV RNA after treatment course), withdrawal (stopped treatment due to adverse effects or no response), no response (positive HCV RNA in all duration of treatment course), breakthrough (relapsed before the end of treatment course), death (death during treatment course).
As proportion of genotype-1 in naïve subgroup (no previous treatment) was not similar to Iranian population, and both cost and effectiveness outcomes are strongly dependent to genotype distribution of patients, comparing these two strategies in this subgroup was not relevant and valuable, therefore we did not calculate and report cost effectiveness ratios for this subgroup. Patients with genotype-1 virus, who consist about 66% of these patients population in Iran, have a much lower probability of SVR than those patients with non-genotype-1 virus (20).
3.2.2. Cost
In this model, only direct medical costs including medication (pharmaceutical) costs, laboratory tests, costs of side effects treatment and physician visits were taken into account and direct non-medical costs, indirect costs and intangible costs were not included in our analysis. Costs of physician visit charges and laboratory tests were extracted from the annual report of Medical Council of Islamic Republic of Iran. Costs of medicines were extracted from the Ministry of Health annual report on pharmaceutical market entitled “Amarnameh.” To include side effect costs into our analysis, we only took physician visits and major medical and laboratory tests into account and the pharmaceutical cost were ignored because their costs were not considerable. The cost analysis was based on cost data for 2008. We also used 9300 Iranian Rials (IR Rial) as exchange rate declared by the Iranian central bank on that time to calculate costs by US Dollar (USD). No discount rate was considered in the cost analysis of this study.
Considering the genotype distribution of different subgroups in the patients, the weighted mean cost for each subgroup and also weighted mean cost of 100 patients were calculated.
3.3. Cost Effectiveness Ratios
Two cost effectiveness ratios were calculated based on our decision tree: cost effectiveness ratio (CER) which means cost per one SVR and indicates superiority of one strategy to the other one for cost effectiveness; and incremental cost effectiveness ratio (ICER) which means cost per one more SVR. To evaluate whether one strategy with more cost and more effectiveness or with less cost and less effectiveness is cost effective, we compared the ICER with recommended threshold of the World Health Organization (WHO) for countries without any calculated threshold. In this method, ICER has to be compared with one and three times of GDP per capita and if it was less than one time of GDP per capita, it would be “highly cost effective” and if it was between one time and three times of GDP per capita it would be “cost effective”, but if it was more than three times of GDP per capita, it is “non-cost effective” (21). 4,678 USD was used as GDP per capita of Iran in 2008 based on the World Bank reports. This recommendation of WHO about threshold is mostly for cost utility analysis with “cost per QALY gained” or “cost per DALY averted” as outcome but as there is not any threshold calculated or accepted for Iran, we used it as our threshold.
3.4. Sensitivity Analysis
A deterministic one-way sensitivity analysis was conducted to evaluate the sensitivity of the result to the changes in key input variables of the model. The considered key variables in this study were SVR and Positive Predictive Value (PPV) of EVR for combination therapy. PPV of EVR is defined as the proportion of EVR patients (complete or partial) with treatment success at the end of study. PPV could be associated with several internal and external parameters including sensitivity and specificity of laboratory tests, pretest probability, quality of medicines, HCV genotype and patients characteristics. By considering the minimum domain of 95% confidence interval of SVR and PPV, the ICER and also CER would be calculated to see how much the results of our model is dependent to variability of key input parameters regarding their uncertainties.
4. Results
SVR and PPV were calculated for two treatment strategies which are showed in the Table 1. As seen in this table, PPV is clearly higher in combination therapy strategy for all subgroups. This means that higher percent of monotherapy group patients, would not achieve SVR and higher cost for each SVR would be imposed to healthcare system and patients.
| Subgroup | Strategy | EVRa | SVRa (95% CIa) | PPVa (95% CI) |
|---|---|---|---|---|
| Genotype-1 | PEG IFN alfa-2a | 84.85 | 29.27% (15.34 – 43.20%) | 39.29 (21.20-57.38) |
| PEG IFN alfa-2a + RBVa | 93.00 | 48.74% (39.72 – 57.76%) | 59.14 (49.15-69.13) | |
| Genotype non-1 | PEG IFN alfa-2a | 90.91 | 47.50% (32.02 – 62.98%) | 53.33 (35.48-71.19) |
| PEG IFN alfa-2a + RBV | 96.97 | 54.43% (43.45 – 65.41) | 59.38 (47.34-71.41) | |
| Low Viral Load | PEG IFN alfa-2a | 84.21 | 39.13 (25.03 – 53.23) | 40.63 (23.61 – 57.64) |
| PEG IFN alfa-2a + RBV | 96.88 | 54.87 (45.69 – 64.04) | 60.22 (50.27 – 70.16) | |
| High Viral Load | PEG IFN alfa-2a | 92.59 | 38.71 (21.56 – 55.86) | 52.00 (32.42 – 71.58) |
| PEG IFN alfa-2a + RBV | 91.18 | 44.71 (34.14 – 55.28) | 59.68 (47.47 – 71.89) | |
| Naïve | PEG IFN alfa-2a | 91.43 | 41.86 (27.11 – 56.61) | 50 ( 28.61 – 71.39) |
| PEG IFN alfa-2a + RBV | 95.92 | 49.18 (36.63 – 61.73) | 57.45 (36.30 – 78.59) | |
| Resistant to Previous Monotherapy | PEG IFN alfa-2a | 85.71 | 41.67 (21.94 – 61.39) | 55.56 (34.30 – 76.81) |
| PEG IFN alfa-2a + RBV | 96.36 | 59.68 (47.47 – 71.89) | 69.81 (57.45 – 82.17) | |
| Resistant to Previous Combination Therapy | PEG IFN alfa-2a | 57.14 | 21.43 (0 – 42.92) | 12.5 (3.6 – 21.4) |
| PEG IFN alfa-2a + RBV | 91.94 | 44.59 (33.27 – 55.9) | 54.39 (40.98 – 67.80) |
a CI, Confidence Interval; EVR, Early Virological Response; PPV, Positive Predictive Value; RBV, Ribavirin; SVR, Sustain Virological Response
Table 2 indicates the cost of different components of HCV treatment in thalassemia major patients in Iran. As it is shown, there is not any considerable difference in medication (pharmaceutical) costs between these two treatment strategies in Iran because Ribavirin is far less expensive than PEG IFN alfa-2a.
| Service | Detail | Cost (USDa) |
|---|---|---|
| Medication Cost | ||
| Pegasys Injection | Pegasys dose was 180 μg/week | 290 |
| Ribavirin rid Tablet 200 mg | Ribavirin dose for Hb < 10 and Hb > 10 was 600 mg/day and 800 mg/day, respectively | 0.34 |
| Visits And Hospitalization | ||
| Routine Visits | Gastroenterologist (totally 16 times in treatment duration) | 7.4 |
| Consult Visits | Cardiologist, psychiatrist, ophthalmologist and etc. (for adverse effects) | 7.4 (9.1 for psychiatrist) |
| Hospitalization | Due to sepsis, DKA, heart failure, severe depression, hepatic decompensation | Mean cost: 215 |
| Laboratory Tests | ||
| Enrollment Tests | Include CBC, TFT, LFT, biochemistry, hepatitis serology, liver biopsy, PCR, lipid profile, LDH, AFP and genotyping) | 325.75 |
| Routine Safety Evaluation Tests | CBC, LFT, Ferritin, TG, Cholesterol and FBS | 8.3 |
| Occasionally Tests | HCV viral load in third month and PCR in sixth and 12th months of treatment course and third and sixth months of follow up course | 139.7 |
| Thyroid function test (TFT) each 3 months | 15 | |
| CBC | It was performed weekly until the third month, then 2 times per month until 12th month and monthly in follow up course for combination therapy group. | 0.6 |
| Cost of Supportive Intervention for Adverse Effects | ||
| Packed Cell | Monotherapy and combination therapy patients' need to packed cell was averagely 33 and 54 units respectively. | 129 |
a Abbreviations: USD, US Dollar
The outcomes of cost effectiveness analysis were calculated for combination and monotherapy strategies in the time period of 72 weeks and are summarized in Table 3.
| Subgroup | Strategy | SVRb(95%CI) | Cost for 100 Patients (USD) | CERb(USDbper one SVR) | ICERb(USD per one more SVR) |
|---|---|---|---|---|---|
| Genotype-1 | PEG IFN alfa-2a | 29.27% (15.34 – 43.20%) | 1,754,180 | 59,934 | 2,673 |
| PEG IFN alfa-2a + RBVb | 48.74% (39.72 – 57.76%) | 1,806,231 | 37,058 | ||
| Genotype non-1 | PEG IFN alfa-2a | 47.50 (32.02 – 62.98) | 1,171,742 | 24,668 | 19,210 |
| PEG IFN alfa-2a + RBV | 54.43% (43.45 – 65.41) | 1,304,872 | 23,973 | ||
| Low viral load | PEG IFN alfa-2a | 39.13 (25.03 – 53.23) | 1,536,399 | 39,263 | 5,233 |
| PEG IFN alfa-2a + RBV | 54.87 (45.69 – 64.04) | 1,618,766 | 29,503 | ||
| High viral load | PEG IFN alfa-2a | 38.71 (21.56 – 55.86) | 1,482,614 | 38,300 | 14,975 |
| PEG IFN alfa-2a + RBV | 44.71 (34.14 – 55.28) | 1,572,469 | 35,173 | ||
| Naïvec | PEG IFN alfa-2a | 41.86 (27.11 – 56.61) | 1,733,217 | NA | NAb |
| PEG IFN alfa-2a + RBV | 49.18 (36.63 – 61.73) | 1,690,536 | NA | ||
| Resistant to Previous Monotherapy | PEG IFN alfa-2a | 41.67 (21.94 – 61.39) | 1,683,906 | 40,413 | 13,005 |
| PEG IFN alfa-2a + RBV | 59.68 (47.47 – 71.89) | 1,918,141 | 32,141 | ||
| Resistant To Previous Combination Therapyd | PEG IFN alfa-2a | 21.43 (-0.07 – 42.92) | 1,939,034 | NA | NA |
| PEG IFN alfa-2a + RBV | 44.59 (33.27 – 55.92) | 1,807,838 | NA |
a GDP per capita: 4,678 USD; 3 times of GDP per capita:14,034 USD
b Abbreviations: CER, Cost Effectiveness Ratio; ICER, Incremental Cost Effectiveness Ratio; NA, Not Applicable; RBV, Ribavirin; SVR, Sustain Virological Response; USD: US Dollar.
c In this subgroup, the proportion of genotype-1 was not similar to Iranian population so we did not report any ratio for them
d In this subgroup, combination therapy was a dominant strategy (higher effectiveness with lower cost) so calculation of cost effectiveness ratios was not reported.
Patients with genotype-1: the CER for monotherapy and combination therapy was 59,934 and 37,058 USD per SVR, respectively. So combination therapy with PEG IFN alfa-2a and Ribavirin needed less cost to achieve SVR. The calculated ICER for combination therapy was 2,673 USD per SVR that is less than GDP per capita. It means that, adding Ribavirin to PEG IFN alfa-2a could be considered as “highly cost effective” strategy in genotype-1 patients.
Patients with genotype non-1: CER for monotherapy and combination therapy was 24,668 and 23,973 USD per SVR, respectively. So combination therapy with PEG IFN alfa-2a and Ribavirin needed less cost to achieve SVR. But ICER for combination therapy was 19,210 USD per SVR which was higher than three times of GDP per capita implying that adding Ribavirin to PEG IFN alfa-2a does not seem to be a “cost effective” strategy for these genotypes in Iran.
Low basic viral load: CER for monotherapy and combination therapy was 39,263 and 29,503 USD per SVR, respectively. So combination therapy with PEG IFN alfa-2a and Ribavirin needed less cost to achieve SVR. The ICER for combination therapy was 5,233 USD per SVR that was lower than three times per capita GDP. So adding Ribavirin to PEG IFN alfa-2a could be “cost effective.”
High basic viral load: CER for monotherapy and combination therapy was 38,300 and 35,173 USD per SVR, respectively. So combination therapy with PEG IFN alfa-2a and Ribavirin needed less cost to achieve SVR. But ICER for combination therapy was 14,975 USD per SVR which was higher than three times of GDP per capita so adding Ribavirin to PEG IFN alfa-2a could not be considered as a “cost effective strategy.”
Naïve patients: In this subgroup, the proportion of genotype-1 patients in the sample was not similar to society so we could not compare two strategies because of different duration of treatment and useless result for policy makers in Iran.
Resistant to previous monotherapy: CER for monotherapy and combination therapy was 40,413 and 32,141 USD per SVR, respectively. So combination therapy with PEG IFN alfa-2a and Ribavirin needed less cost to achieve SVR. The ICER for combination therapy was 13,005 USD per SVR which was lower than three times per capita GDP so adding Ribavirin to PEG IFN alfa-2a in this category of patients is a “cost effective” strategy in Iran.
Resistant to previous combination therapy: In this subgroup, combination therapy was a dominant strategy meaning that it had more effectiveness and less cost compared to monotherapy which did not need any economic evaluation.
4.1. Sensitivity Analysis
For sensitivity analysis, we calculated the ICER with the minimum amount of SVR and PPV in their 95% confidence interval. In minimum amount of PPV, combination therapy in genotype non-1 would be dominated and ICER in all other groups would be higher than three times of GDP per capita. In minimum amount of SVR in 95% confidence interval, combination therapy in genotype non-1 and high viral load subgroups would be dominated and in all other subgroups ICER would be higher than three times of GDP per capita. In Table 4, the calculated CER and ICER with minimum amount of SVR and PPV in several subgroups are shown.
| Subgroup | Monotherapy | Combination Therapy | |||
|---|---|---|---|---|---|
| CERb (USDbper SVR) | Minimum PPVbin 95%CI | Minimum SVRbin 95%CI | |||
| CER (USD per SVR) | ICERb(USD per one more SVR) | CER (USD per SVR) | ICER (USD per one more SVR) | ||
| Genotype-1 | 59,934 | 39,523 | 316,773 | 45,473 | 497,982 |
| Genotype non-1 | 24,668 | 28,428 | Dominated | 30,033 | Dominated |
| Low Viral Load | 39,263 | 33,239 | 860,725 | 35,427 | 1,255,309 |
| High Viral Load | 38,300 | 36,332 | 1,966,050 | 46,064 | Dominated |
| Resistant to Previous Monotherapy | 40,413 | 34,654 | 1,711,831 | 40,410 | 4,038,486 |
a GDP per capita: 4,678 USD; 3 times of GDP per capita:14,034 USD.
b Abbreviations: CER, Cost Effectiveness Ratio; ICER, Incremental Cost Effectiveness Ratio; PPV, Positive Predictive Value; SVR, Sustain Virological Response; USD, US Dollar.
5. Discussion
This study indicated that although in all subgroups, combination therapy was more effective than monotherapy, but combination therapy of HCV in thalassemia major patients could be considered as a “highly cost effective” strategy only in genotype-1 patients and as a “cost effective” strategy in low basic viral load patients and also in patients with previous resistance to monotherapy. It was shown that in patients with previous resistance to combination therapy, it is a dominant strategy to put them therapy again in comparison with monotherapy.
The other studies comparing monotherapy and combination therapy regimens in hepatitis C patients have shown that combination therapy is the most cost effective treatment in the patients with genotypes 2, and 3 and low viral load patients (21-24); however, our study indicated that in HCV infected thalassemia major patients it was for the patients with genotype-1 and low viral load patients and previous monotherapy resistant patients. One limitation of our study was that the proportion of genotype-1 was not similar to HCV infected thalassemia major population of Iran in naïve subgroup; therefore we were not able to generalize the result of this study to Iranian setting whether combination therapy is cost effective in this subgroup. As this is an applied study to be used only by policy makers in Iran, we decided not to calculate and report the ICER for this subgroup.
This analysis was based on the clinical trial evaluating both monotherapy and combination therapy group with the same inclusion criteria. As the types and severity of adverse events experienced by patients who were exposed to PEG IFN alfa-2a and Ribavirin might be varied, we tried to include consideration of adverse events which could be resulted from treatment in the analysis. Using clinical data from a domestic clinical trial could be considered as an advantage for this study because of more similarity with real setting of Iran, but has its own weaknesses. One limitation was that only one domestic clinical trial was found which was not multicenter, however we tried to overcome this limitation by sensitivity analysis. The number of patients in this clinical trial was also another limitation especially when we categorized them into subgroups which caused relatively wide 95% confidence interval. Another limitation of this study was that we did not take patients' quality of life into consideration. In one study evaluating the effects of PEG IFN alfa-2a plus Ribavirin in patients with chronic hepatitis C on their quality of life, the results indicated that quality of life of these patients was significantly improved (25). Using more comprehensive outcome of interest (i.e. quality adjusted life years (QALY) or disability adjusted life years (DALY)) could provide better understanding about different aspects of disease and treatment effects. Also using more complicated decision support models including Markov models which consider more details of disease could result in more accurate output for policy makers.
This study was based on cost data of 2008, so considering much market variability in the health sector of Iran in the last years, the results may not be easy to defend in 2013. However it is a challenge over economic evaluation studies in the countries with rapidly changing or unstable economic situation.
Although both medicines are available in Iran, this study is the first economic evaluation of two common treatment strategies for HCV infection in thalassemia patients. The results of this analysis could be used by policy makers in setting reimbursement strategies and guideline development. More analysis is suggested to be conducted by considering more domestic and also international evidences, using more complicated models and more comprehensive outcomes and perspective for more accurate results. This study indicated that adding low dose of Ribavirin to PEG IFN alfa-2a for treatment of chronic hepatitis C infection in thalassemia is highly cost effective in genotype-1 patients and also cost effective in patients with low viral load or with previous monotherapy resistance. But in patients with genotype non-1 or high viral load, adding Ribavirin to PEG IFN alfa-2a could not be considered as a cost effective strategy.