1. Context
Non-alcoholic steatohepatitis (NASH), recently renamed metabolic dysfunction-associated steatohepatitis (MASH), is a clinical condition linked to non-alcoholic fatty liver disease (NAFLD) that arises due to metabolic dysfunction of the liver, leading to cancer and cirrhosis (1). Globally, the average prevalence of NASH is between 1.5% and 6.45% (2). This condition may further progress to liver fibrosis, hepatocellular carcinoma (HCC), and cirrhosis in 30% to 40% of patients (3-6), and 20% suffer from advanced fibrosis, known as bridging fibrosis (F3) or cirrhosis (F4) (7, 8). However, the diagnosis of fibrosis is visualized over the years after the onset of the disorder, and it remains a predictive marker to map the severity and incidence of mortality and morbidity (9).
Despite no approved biomarker for NASH, traditional serum biomarkers, including total cholesterol, triglycerides, insulin resistance, C-peptide, apolipoprotein A1, apolipoprotein B, leptin, adiponectin, free fatty acids, ghrelin, and cytokeratins, are increasingly recognized as important predictors of disease risk (10). The NASH could be a multifactorial metabolic syndrome clinically diagnosed as adiposity in the visceral, with imbalanced metabolism resulting in stress-related inflammation leading to fibrosis (11). Focusing on the pathophysiology of NASH relates to the multifactorial pathway where toxic hepatocyte cells release immune-mediated inflammation signals, playing a key role in causing higher prevalence in patients with obesity and type 2 diabetes mellitus (T2DM) (12, 13).
The primary mechanism explaining the inflammation and liver damage in NASH is insulin resistance; these individuals exhibit elevated blood glucose levels that increase fatty acids, resulting in the accumulation of fat in liver cells that progress to cirrhosis and other complications like diabetic retinopathy, chronic kidney disease, and cardiovascular disease (14). Hence, given all these mechanistic aspects, focusing on potential drug inhibitors that act on hepatic enzymes and regulate fat, consequently improving the function of renal tubules through reabsorption of glucose and eliminating the increasing urinary glucose, has become essential to improve the clinical condition of NASH (15).
There is no approved medication for NASH; however, significant efforts have been made in research to identify a drug that can be tailored to control NASH. Sodium-glucose transport protein-2 (SGLT-2) inhibitor drugs efficiently lower glucose levels in the blood and address associated metabolic comorbidities, such as renal/kidney outcomes and cardiovascular-related conditions (16). Experimental evidence has shown that ipragliflozin directly correlates with enhancing the concentration of FGF21, a key hepatokine with beneficial properties, thus significantly improving oxidative stress, inflammation, and fibrosis (17, 18).
The mechanism of SGLT-2 inhibitors in the treatment of NASH is not fully understood; however, by administering these drugs, the rationale function of SGLT-2 targets the diseased organ to reduce accumulated fat by losing calories and improving insulin resistance. This is achieved by enhancing functional gluconeogenesis in pancreatic α-cells and inducing β-oxidation by suppressing Kupffer cell activation (19). Open-label studies and randomized controlled trials have recently demonstrated the effect of SGLT-2 inhibitors in gradually reducing liver fat and improving patient conditions (20).
Studies such as the CREDENCE trial, CVD-REAL study, EMPA-REG outcome, CANVAS program (21), DAPA-HF study, DECLARE trial (22), and EMPEROR-Reduced trials have accumulated evidence on SGLT-2 inhibitors, showing improved levels of ALT and hepatoprotective steatosis effects used to treat patients with heart failure, with or without diabetes (23, 24).
2. Objective
This review aims to assess available data from published literature on various SGLT-2 inhibitor drugs for treating NASH and their beneficial effects on protecting the liver from further damage and progression to cirrhosis.
3. Methods and Discussion
3.1. Data Collection and Search Strategy
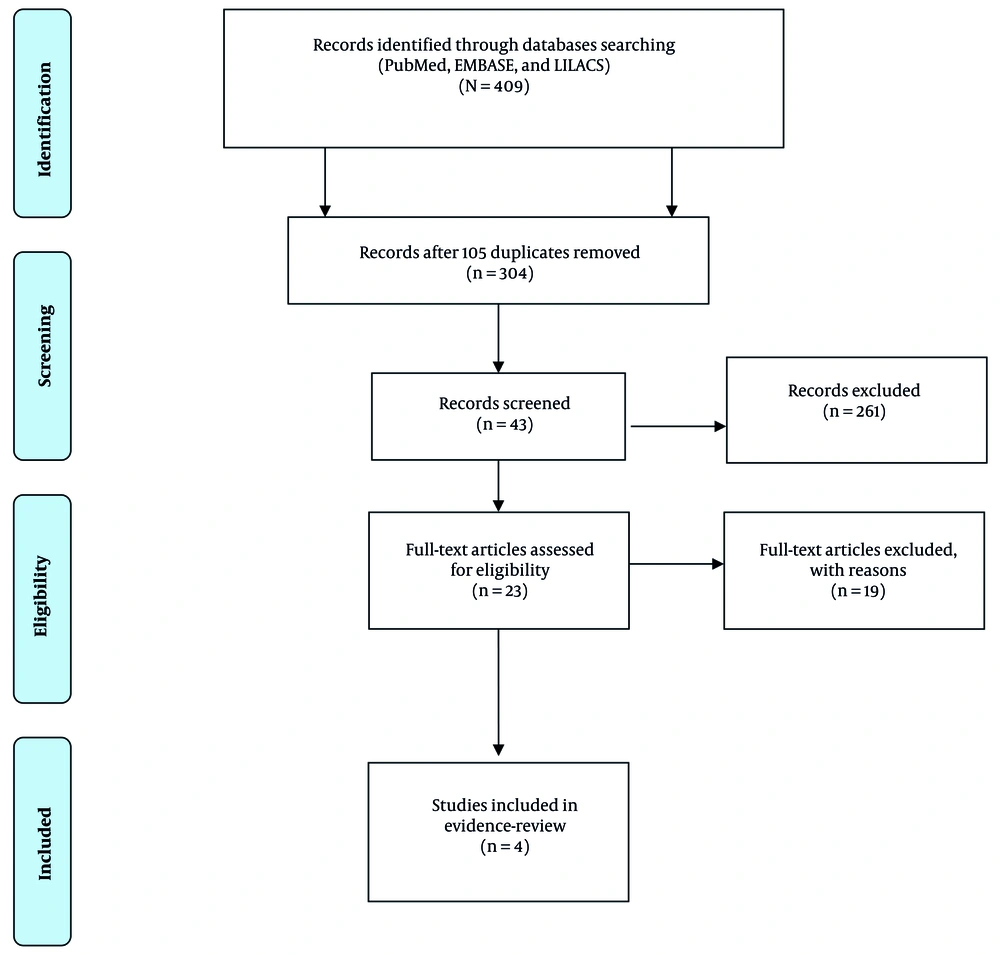
In this evidence-based review, published literature was obtained only from studies involving patients with NASH who received SGLT-2 inhibitors. To obtain the required information, we screened published studies from January 2014 to June 2024 (Figure 1). We conducted a detailed literature search using the following databases: PubMed, EMBASE, and LILACS. The literature search was performed to find suitable articles using MeSH search terms: "SGLT-2 inhibitor" AND "NASH", "SGLT-2" AND "Nonalcoholic Steatohepatitis", "Canagliflozin" AND "Nonalcoholic Steatohepatitis", "Dapagliflozin" AND "Nonalcoholic Steatohepatitis", "Empagliflozin" AND "Nonalcoholic Steatohepatitis", "dapagliflozin/exp" OR "dapagliflozin" AND "non-alcoholic steatohepatitis/exp" OR "non-alcoholic steatohepatitis" OR "non-alcoholic" AND "steatohepatitis/exp" OR "steatohepatitis", "canagliflozin/exp" OR "canagliflozin" AND "non-alcoholic steatohepatitis/exp" OR "non-alcoholic steatohepatitis" OR "non-alcoholic" AND "steatohepatitis/exp" OR "steatohepatitis" AND "Nonalcoholic Steatohepatitis", "Bexagliflozin" OR "Nonalcoholic Steatohepatitis", "Ertugliflozin," OR "Nonalcoholic Steatohepatitis", "licogliflozin", OR "Nonalcoholic Steatohepatitis". This evidence-based review has been registered on the OSF database (DOI identifier: https://doi.org/10.17605/OSF.IO/V2Y5N).
3.2. Inclusion and Exclusion Criteria
The inclusion criteria for selection included (1) randomized controlled trials and open-label studies with participants diagnosed with NAFLD/NASH; and (2) the use of SGLT-2 inhibitors. Non-randomized clinical trials, cohort studies, case-control studies, cross-sectional studies, and case reports were excluded. There were no limitations regarding the type of setting and length of follow-up.
3.3. Data Collection Process
Two independent reviewers thoroughly checked the articles to determine whether the content matched the selected title. Reviewers were paired based on their education, with each pair consisting of one person with clinical expertise and one with research experience. The reviewers initially understood the inclusion and exclusion criteria, which helped in selecting only those screened titles and abstracts of relevant articles from the database. The full text of relevant articles was retrieved from the database. The two reviewers independently reviewed the same article and provided individual results. If any discrepancies were found concerning the study eligibility criteria, they were brought to the attention of the study team for a final decision.
3.4. Data Extraction
Data were reviewed and obtained from the final list of articles, which was further verified by the group of reviewers. The team recommended including the following data items: The objective of the study, study design, inclusion and exclusion criteria, sample size, treatment given to the subjects, and results obtained. It was mandatory to follow the protocol because any deviations from the study design would result in the committee not approving the study.
3.5. Dapagliflozin
Dapagliflozin is a recommended drug for T2DM, as it shows beneficial effects in patients with T2DM and fatty liver. The usual recommended dose is 5 mg/day to 10 mg/day for 24, 28, and 52 weeks, depending on the severity of the condition. In some trials, it has exhibited combined effects when used with another drug without any adverse effects. Tobita et al., in their study, revealed that with 5 mg/day dapagliflozin, there are several health benefits if administered for 24 weeks (25). After 24 weeks of treatment, a statistically significant reduction (P < 0.01) in waist-to-hip ratio, Body Mass Index (BMI), and waist circumference was observed. Similarly, changes in body composition also showed reduced fat (P < 0.01) and fat percentage (P < 0.01), with no changes in total body water or lean mass. For the study, liver tests corresponding to serum concentrations of type IV collagen 7S, alanine aminotransferase, aspartate aminotransferase, and ferritin levels were significantly improved. By the 24th week, there was a significant decrease in insulin concentrations (P < 0.01), specifically glycated hemoglobin (P < 0.01) and fasting plasma glucose (P < 0.01). From the beginning of the 4th week, increased adiponectin levels (P < 0.01) were found. Therefore, dapagliflozin was considered an effective drug in patients with NASH and T2DM, as it reduced visceral fat, reflecting improved changes in metabolic variables.
In another study, a similar dose of 5 mg/day dapagliflozin was administered daily for 24 weeks, and an improvement in liver steatosis and fibrosis was found. Liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) gradually decreased from 14.7 ± 5.7 to 11.0 ± 7.3 kPa (P = 0.0158) and 314 ± 61 to 290 ± 73 dB/m (P = 0.0424), respectively (Table 1).
| Study | Study Design | Treatment Drug vs. Comparator | Study Duration (wk) | Study Participants | Outcome Results | Safety Results |
|---|---|---|---|---|---|---|
| Tobita et al. (2017) (25) | Prospective, open-label, uncontrolled pilot study | Dapagliflozin 5 mg/day; no comparator | 24 | 16 patients with biopsy-confirmed NASH and T2DM; 11 completed the study. | Significant improvement in BMI, waist circumference, liver tests (AST, ALT), and metabolic parameters (HbA1c, insulin) | Two participants discontinued due to adverse effects (severe hunger, epigastric discomfort). Overall, well tolerated with no severe adverse effects. |
| Harrison et al. (2022) (26) | Randomized, double-blind, placebo-controlled, Phase 2a | Licogliflozin 30 mg and 150 mg vs. placebo | 12 | 107 patients with phenotypic or histologic NASH; 96 completed the study. | Licogliflozin 150 mg significantly reduced ALT, AST, and liver fat content. Improvements in markers of liver fibrosis (ELF scores) were observed in some patients. | Diarrhea was the most frequent adverse event (77% at 150 mg dose), mostly mild. No major safety concerns were identified. |
| Lai et al. (2020) (27) | Single-arm, open-label pilot study | Empagliflozin 25 mg/day vs. historical placebo group | 24 | 9 patients with biopsy-proven NASH and T2DM | Significant reductions in liver fat fraction, BMI, steatosis, ballooning, and fibrosis scores. Improvements were greater compared to historical placebo. | Well tolerated; no severe hypoglycemia or genitourinary infections reported. Adverse events were not clinically significant. |
| Seko et al. (2018) (28) | Single-arm, exploratory study | Canagliflozin 100 mg/day; no comparator | 12 | 10 patients with biopsy-proven NASH (fibrosis stages 1 - 3) and T2DM | Improvements in ALT, AST, liver fibrosis markers (FIB-4 Index, FM-Fibro Index), and body weight. Effects were more pronounced in early-stage NASH. | No serious or liver-related adverse events reported. Safe and well-tolerated. Differences in ALT-lowering effects were observed between fibrosis stages. |
Sodium-Glucose Transport Protein-2 Inhibitors and Their Clinical Effect in Patients with Non-alcoholic Steatohepatitis
Clinical studies on dapagliflozin for NASH treatment have found that almost all have conferred similar significant results concerning treatment outcomes after recommending dapagliflozin, even though the dose range varied from 5 mg to 10 mg, given as monotherapy or combination therapy between 24 to 28 weeks, or extending to 52 weeks. Administration of this drug has shown better results in visceral fat reduction, BMI management, improvement in associated T2DM conditions, and decreases in liver fibrosis-LSM, γ-glutamyl transpeptidase levels, serum alanine aminotransferase, FIB-4, and FIL. To support these study results, it is essential to understand the mechanism of the drug in treating or reducing/improving the coexisting biochemical levels for proper metabolism that triggers the cure of NASH and other existing comorbidities.
From preclinical and clinical studies, it is known that dapagliflozin for NASH treatment has remained beneficial in improving the patient's clinical condition. However, to strengthen the study, it is necessary to understand that a mutual interplay exists between diabetes mellitus and NAFLD/NASH, which gradually leads to comorbidities like cirrhosis, hepatic fibrosis, and hepatocellular carcinoma (29). It is understood from experimental models that metabolic imbalance is significantly associated with the development of NASH, aside from DM.
Leng et al. (30), in their study on dual HFD/STZ-treated ApoE (–/–) mice, explained that treatment with dapagliflozin reduced inflammation due to hepatic rupture, gradually lowering liver fibrosis and steatosis, blood glucose, and majorly the accumulation of fat in the liver. A probable mechanism could be the minimal production of malondialdehyde (MDA) and hepatic reactive oxygen species (ROS) and suppression of the stimulation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome.
In another study, the efficiency of dapagliflozin was assessed on selective T2DM C57BL/6NCrl mice liver mitochondria induced by HFD/STZ injections, which normalized the swelling mitochondrial respiratory control ratio in hepatocytes and increased the mtDNA copy number and enhanced the Mfn2, Ppargc1a, and Drp1 expression in liver tissue. Consequently, there was a gradual reduction in transition pore permeability and accumulation of lipid peroxidation products (31).
The probable mechanism involved could be explained through the balance of negative energy by energy loss via urine and increased oxidation of fatty acids (32), which would be elevated by an increased glucagon or insulin ratio (33). When dapagliflozin is given with OM-3CA, it possibly increases the transcription of transaminases through peroxisome proliferator-activated receptor α via OM-3CA. It also reduced a considerable amount of PDFF, which is regulated by increased oxidation of fatty acids, thereby reducing fatty acid synthesis (34).
3.6. Canagliflozin
Canagliflozin (100 mg) once a day for 12 weeks is the most regularly recommended dose as a course of treatment. Seko et al. (28) observed a significant improvement in uric acid metabolism (P = 0.0016) by the end of 12 weeks, with a reduction of -1.06 U/L (95% CI -1.60 to 0.52), compared to other metabolic and hepatic markers, which gradually improved liver function (P < 0.05) (Table 1).
Canagliflozin, another member of the SGLT-2 inhibitors, can eliminate more glucose through urine to maintain blood sugar levels and lower the risk of hepatic burden. Significantly, six studies were noted, and we attempted to extract the possible mechanisms interplaying to safeguard the organs by preventing the emergence of comorbidities and NASH. This was better explained in a study where Western diet (WD)-fed melanocortin 4 receptor-deficient (MC4R-KO) mice, a model for testing human NASH, were administered canagliflozin for eight weeks, which attenuated the process of hepatic steatosis (35). However, the epididymal fat mass remained increased even without inflammatory reactions. When extended for 20 weeks, CANA treatment inhibited hepatic fibrosis in WD-fed MC4R-KO mice. After 1 year, liver tumors gradually reduced in WD-fed MC4R-KO mice. It also suppressed the oxidative proportion to reduce glutathiones (GSSG/GSH) in WD-fed MC4R-KO mice, greatly influencing glutathione metabolism in the epididymal fatty tissue to reduce oxidative stress, as assessed by the ratio of GSSG to GSH. This suggests activation of insulin signaling, which reduces the GSSG/GSH ratio. Hence, this study in mice provides evidence that CANA is unique among other SGLT2 inhibitors in diminishing or delaying the onset of NASH, which might eventually restrict the occurrence of hepatocellular comorbidities, at least partly, through "healthy adipose expansion".
The most established mechanism available for this drug in various studies is to improve the hepatic steatosis clinical condition by losing energy through the elimination of urinary glucose, visceral fat, and body weight reduction, gradually improving liver metabolism by enhancing β-oxidation activity. However, some results are inconsistent with previous studies showing improvement in hepatic steatosis, visceral fat mass, and body weight (36, 37).
The second benefit of SGLT2 inhibitors is that they lower blood glucose and insulin resistance (38). This process downregulates the binding affinity of carbohydrate-responsive element-binding protein (ChREBP), a transcription factor for liver fatty acid synthesis, and sterol regulatory element-binding protein 1c (SREBP-1c). This transcription factor suppresses de novo liver lipogenesis (39). The third outcome of SGLT2 inhibitors is that they suppress glucose oxidation, inflammatory markers, and oxidative stress; simultaneously, they accelerate free fatty acid oxidation and lipolysis (40). These changes may help improve NAFLD.
3.7. Empagliflozin
Empagliflozin has established its role in treating liver steatosis, NASH, and T2DM. The two probable doses recommended to patients are 10 mg for 20 weeks and 25 mg for 24 weeks. We focused on a recent study that resulted in positive outcomes with empagliflozin, even with a low dose and fewer weeks of intervention. Lai et al. (27) found significant results with a dose of 25 mg of empagliflozin for 24 weeks versus a historical placebo. A reduction was found in BMI and total cholesterol (P = 0.011); waist circumference and diastolic blood pressure significantly improved (P = 0.033), while systolic blood pressure had a P-value of 0.024. A tremendous improvement was observed in fasting blood glucose, with a P-value of 0.008. Further, gamma-glutamyl transpeptidase (P = 0.013) and steatosis had a significant reduction with a P-value of 0.014, followed by a volumetric liver fat fraction with a P-value of 0.017. Slight varying significance was observed with ballooning (P = 0.034) and fibrosis (P = 0.046). Either the histological components remained unchanged or improved. However, ballooning showed a worse effect in one patient. Empagliflozin significantly improved steatosis (P = 0.025), ballooning (P = 0.024), and fibrosis (P = 0.008) compared to the historical placebo. Hence, this study provides a clue about administering lower doses of SGLT-2 drugs for treatment starting from weeks and gradually increasing to months. Although inter-individual variation exists in patients, low doses have confirmed better significant results than higher doses for prolonged weeks; starting with low doses is beneficial to avoid unexpected adverse events or patient dropouts.
This potent antidiabetic restricts the pathogenesis involved in oxidative stress, drug complex, mitochondrial dysfunction, insulin resistance, lipid peroxidation, diabetes, and NAFLD or NASH (41). Experimental studies on ApoE (−/−) mice (42), streptozotocin-induced models (43, 44), OLETF rats, and db/db mice (45) with diabetes and obesity (46) were treated with empagliflozin and showed improved liver steatosis and inflammation caused by macrophage autophagy polarization of the anti-inflammatory M2 phenotype (42, 45, 47), alongside downregulating the genes that activate gluconeogenesis (45), endoplasmic reticulum stress (42, 48), and lipogenesis (42, 46, 48). However, a dose above 200 - 250 mg/dL of empagliflozin has reduced severe glucose levels and body weight. Hence, empagliflozin benefits liver pathology through improvements in the energy homeostasis balance of glucose.
3.8. Licogliflozin
In a randomized, double-blind, placebo-controlled study, patients with NASH (N = 107) treated with licogliflozin 150 mg experienced significant reductions in ALT, AST, and liver fat content. Improvements in markers of liver fibrosis (ELF scores) were also observed (26).
Licogliflozin, a dual SGLT1/2 inhibitor, reduces glucose and galactose absorption in the intestine, promoting calorie loss and weight reduction. It enhances weight loss by lowering postprandial insulin, suppressing appetite, and boosting glucagon-like peptide-1 and peptide YY secretion. Unlike SGLT2-only inhibitors, dual SGLT1/2 inhibition is more effective in reducing weight, particularly in obese patients, potentially improving glucose metabolism, blood pressure, lipids, liver function, and obesity-related complications (49, 50).
This review has some limitations, including the selection of articles in the English language only and the exclusion of other article types.
4. Conclusions
The literature evidence showed some initial promising clinical findings for licogliflozin, empagliflozin, canagliflozin, and dapagliflozin in NASH patients. These drugs could potentially arrest the progression and development of additional comorbidities compared to the available SGLT-2 inhibitors. However, long-term clinical and real-world studies, ideally with 1- to 2-year follow-up periods, are needed to evaluate whether SGLT-2 inhibitors can sustain liver improvements and reduce the risk of advanced fibrosis, cirrhosis, and liver-related death in NASH patients with and without T2DM.