1. Introduction
Talaromycosis is a severe fungal infection caused by Talaromyces marneffei (TM). In 2022, the World Health Organization (WHO) classified TM as a medium-priority pathogen on its inaugural fungal priority pathogen list (1). Infections predominantly occur in Southeast Asia and southern China, primarily affecting individuals with acquired immunodeficiency syndrome (AIDS), anti-interferon gamma autoantibodies (AIGA), primary immunodeficiency diseases, diabetes mellitus, and those receiving glucocorticoids or immunosuppressants. Co-infection with other opportunistic pathogens, along with frequent misdiagnosis and underdiagnosis, significantly impacts patient prognosis (2).
This paper presents a case of Talaromyces marneffei infection, highlighting the clinical presentation, complex diagnostic process, and treatment of a patient primarily presenting with liver failure, who was treated in our department. The objective is to enhance clinicians' understanding of the disease and to encourage rapid and accurate diagnostic investigations, early detection, and timely intervention. Such a strategy aims to reduce misdiagnosis and lower the morbidity and mortality rates associated with this condition.
2. Case Presentation
A 49-year-old male office worker presented to our hospital with persistent symptoms, including a 10-day fever, five days of abdominal distension, and three days of jaundice affecting the skin and sclera. In the prior week, he experienced uncontrolled high fever and received intravenous cefodizime sodium as an anti-infection treatment at local hospitals, but with no improvement. His medical history included a diagnosis of immune thrombocytopenia in March of the previous year, for which he was treated with glucocorticoids. Subsequently, he was diagnosed with diabetes and started insulin therapy to control blood sugar levels. Over the past two months, he reported a weight gain of 10 kg. He denied any history of Hepatitis, drug-induced liver damage, or excessive alcohol consumption.
Upon admission, the patient’s vital signs included a body temperature of 38.8°C, blood pressure of 98/69 mmHg, a pulse rate of 112 beats per minute, and a respiratory rate of 18 breaths per minute. Physical examination revealed decreased orientation, impaired arithmetic abilities, a trance-like state, and pronounced jaundice of the mucous membranes and sclera. The liver, spleen, and superficial lymph nodes were not palpable. The abdomen appeared distended with normal bowel sounds, and the abdominal wall was firm. No tenderness was noted upon abdominal compression, but mobile turbidities were present. Additionally, the patient exhibited noticeable edema in the lumbosacral region and both lower extremities.
| Measure | Reference Range | Patient on Admission | Patient with Acute Liver Failure |
|---|---|---|---|
| White-cell count (10E9/L) | 3.5 - 9.5 | 3.63 | 6.02 |
| Absolute neutrophil count (10E9/L) | 1.8 - 6.3 | 2.88 | 5.03 |
| Absolute lymphocyte count (10E9/L) | 1.1 - 3.2 | 0.36↓ | 0.67↓ |
| Red-cell count (10E12/L) | 4.3 - 5.8 | 2.82↓ | 2.86↓ |
| Hemoglobin (g/L) | 130 - 175 | 83↓ | 83↓ |
| Platelet count (10E9/L) | 125 - 350 | 27↓↓ | 112↓ |
| CRP (mg/L) | 0 - 2.87 | 30.10↑ | - |
| Immunoglobulin G (g/L) | 8.6 - 17.4 | 6.29↓ | - |
| Immunoglobulin A (g/L) | 1.0 - 4.2 | 0.40↓ | - |
| Immunoglobulin M (g/L) | 0.3 - 2.2 | 0.19↓ | - |
| Sodium (mmol/L) | 137 - 147 | 137 | 138 |
| Potassium (mmol/L) | 3.5 - 5.3 | 4.92 | 4.64 |
| Chloride (mmol/L) | 99 - 110 | 111↑ | 99 |
| Calcium (mmol/L) | 2.11 - 2.52 | 2.44 | 2.24 |
| Inorganic phosphorus (mmol/L) | 3.1 - 8.0 | 15.8↑ | 49.7↑↑ |
| Creatinine (μmol/L) | 57 - 97 | 211↑ | 352↑↑ |
| Total protein (g/L) | 65 - 85 | 39.1↓↓ | 44.4↓ |
| Albumin (g/L) | 40 - 55 | 24.5↓↓ | 26.9↓ |
| Alanine aminotransferase (U/L) | 9 - 50 | 323.3↑↑ | 21.1 |
| Aspartate aminotransferase (U/L) | 15 - 40 | 574↑↑ | 379↑ |
| Lactate dehydrogenase (U/L) | 120 - 250 | 1 905.1↑↑ | 2 287.6↑↑ |
| Total bilirubin (μmol/L) | 0 - 26 | 190.8↑↑ | 269.9↑↑ |
| Direct bilirubin (μmol/L) | 0 - 6.8 | 115.2↑↑ | 218.0↑↑ |
| Indirect bilirubin (μmol/L) | 0 - 13.7 | 75.6↑ | 51.9↑↑ |
| Cholinesterase (U/L) | 5 320 - 12 920 | 1 882↓↓ | 5 430 |
| Alkaline phosphatase (U/L) | 45 - 125 | 697↑↑ | 308↑ |
| γ-Glutamyl transpeptidase (U/L) | 10 - 60 | 566.2↑↑ | 144.0↑ |
| International normalized ratio | 1.5 | 1.63↑ | 2.21↑ |
| Prothrombin time activity (%) | 70 - 130 | 49↓ | 35↓↓ |
| Fibrinogen (g/L) | 2 - 4 | 1.45↓ | 0.87↓↓ |
| Blood ammonia (μmmol/L) | 10 - 47 | 92↑↑ | 100↑↑ |
| CD3+% | 56.5 - 85.5 | 80.3 | - |
| CD3+CD45+% | 56.0 - 85.0 | 80.2 | - |
| CD3+CD4+CD45+% | 30.0 - 54.0 | 22.0% | - |
| CD3+CD8+CD45+% | 15.0 - 34.0 | 51.1 | - |
| CD4+CD8+% | 0 - 2 | 0.32 | - |
| CD4/CD8 | 1 - 2 | 0.43 | - |
| CD19+% | 7.3 - 18.2 | 3.0 | - |
| NK cell% | 8.1 - 25.6 | 9.1 | - |
| Procalcitonin (ng/mL) | 0 - 0.052 | 1.684 | 1.372 |
Clinical Laboratory Results
Initial laboratory results upon admission revealed impaired liver function, indicated by significant elevations in AST, ALT, AKP, γ-GT, blood ammonia, TBIL, DBIL, and IBIL. Coagulation disorders were also present, with elevated INR and decreased Fib. Additionally, there were notable alterations in immunological function, including significantly reduced lymphocyte counts, elevated inflammatory markers (C-reactive protein (CRP) and procalcitonin (PCT)), and decreased levels of immunoglobulins (IgG, IgA, IgM), total serum protein, blood albumin, CD4+, and CD4/CD8 ratio. As the etiology of the patient’s liver failure remained unclear, extensive testing was conducted to identify potential causes (Table 1). Results for antinuclear antibodies, autoimmune liver antibodies, and antineutrophil cytoplasmic antibodies were all within normal limits. Tests for Hepatitis A, Hepatitis B, Hepatitis C, HIV, and syphilis were negative. Additionally, tests for Y-interferon, Epstein-Barr virus nucleic acid, and (1,3)-beta-D glucan for fungal infections were negative. In response to the recurring fever, blood and bone marrow cultures were performed but yielded no pathogenic microorganisms.
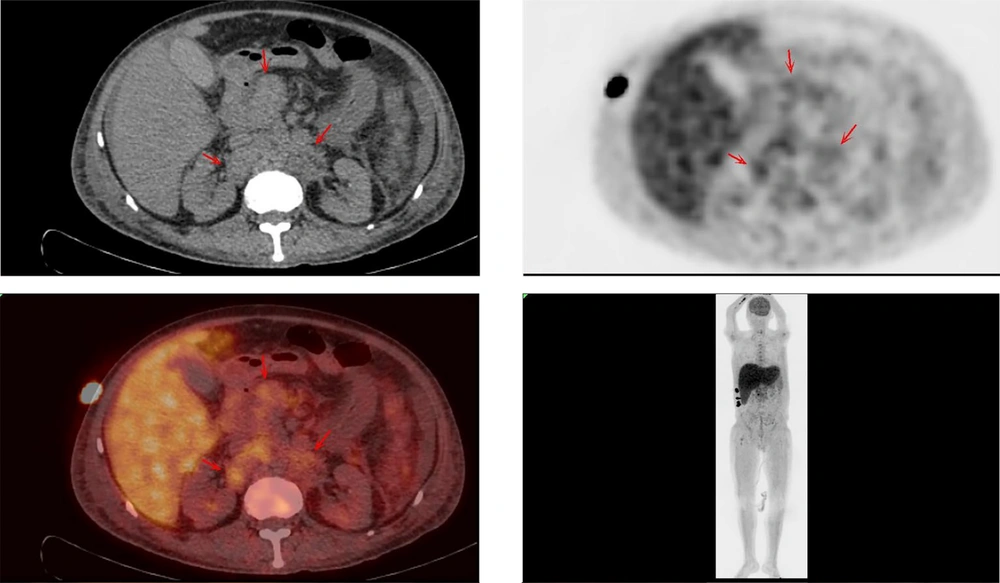
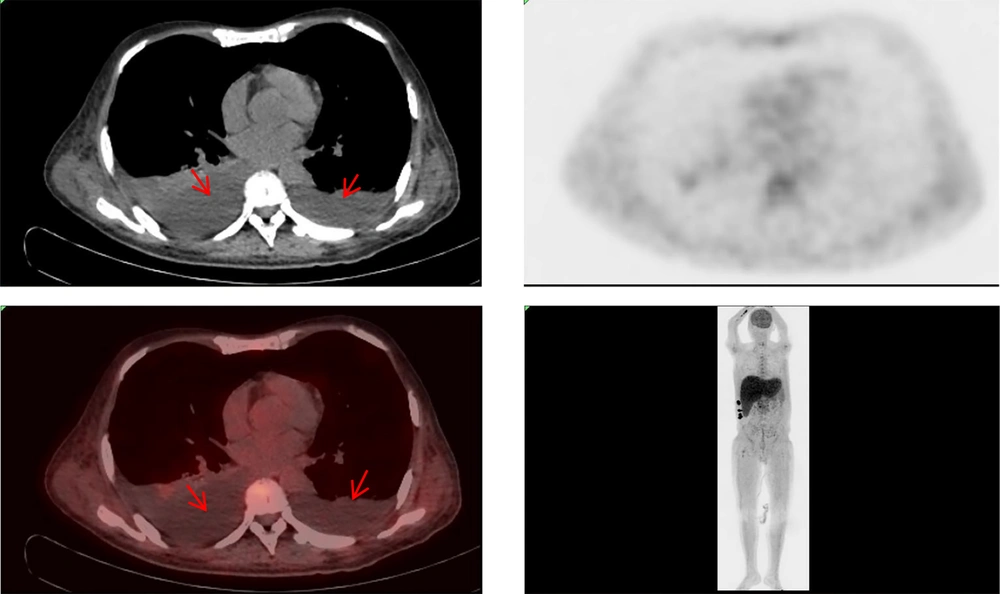
Upon admission, the patient received routine medical management, including antibiotic therapy, liver protection treatments, interventions to reduce bilirubin and blood ammonia levels, supplementation with human albumin, and procedures such as plasma exchange and hemofiltration. Despite these treatments, the patient’s condition failed to improve and progressively worsened. Consequently, thoracic and abdominal computed tomography (CT) scans were conducted, revealing pleural and abdominal effusions as well as multiple enlarged lymph nodes in the abdominal cavity, suggestive of non-draining lymphoma. Further comprehensive whole-body positron emission tomography (PET)/CT imaging was performed, yielding two significant findings:
(1) Multiple enlarged lymph nodes in the hepatic hilar region, hilar hiatus, retroperitoneum, para-abdominal aorta, and right supraclavicular fossa with slightly increased metabolic activity, with some nodes encapsulating adjacent blood vessels, indicating non-draining lymphoma. The maximum standardized uptake value (SUVmax) was 2.70. Abdominopelvic effusion was noted.
(2) Hepatomegaly, hypo-densification, and slightly increased metabolic activity, suggestive of hepatic injury (Figure 1).
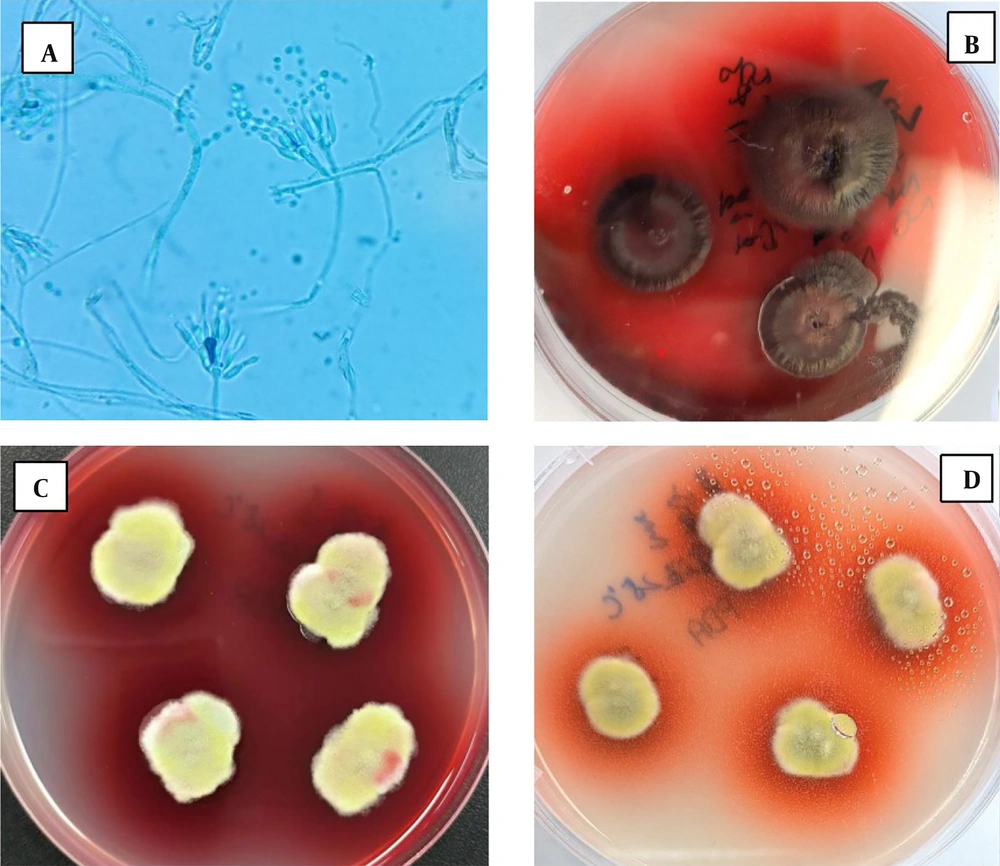
The patient exhibited multiple enlarged lymph nodes within the abdominal cavity, raising suspicion of lymphoma. Consequently, a lymph node biopsy was recommended for pathological examination. However, due to the patient's extremely poor general condition and inability to tolerate general anesthesia and laparoscopic lymph node biopsy, the general surgery department was consulted. As a result, pleural and ascitic fluids were drained (Figure 2), with the pleural fluid sent for culture. Simultaneously, a bone marrow smear was performed, but it revealed no evidence of lymphoma or hematologic disease. Amid these investigations, the patient’s condition rapidly deteriorated. Given the unresolved etiology of the liver failure, the patient was discharged at their request and subsequently passed away. Two weeks later, cultures from the pleural and ascitic fluid isolated TM (Figure 3), a finding that, unfortunately, emerged posthumously.
Results of culture at 25°C. A, Colony smear microscopic examination of conidia (magnification, × 1 000); B, C and D, Pleural and ascitic fluid culture, suggesting TM infection (B, SDA medium, incubated at 25°C for 14 days; C, PDA medium incubated at 25°C for 14 days; D, PDA medium, incubated at 25°C for seven days).
3. Discussion
This Case demonstrates a rare instance of TM infection manifesting primarily as Hepatic failure. The Patient also exhibited multiple complications, including renal failure, anemia, thrombocytopenia, pleural and abdominal effusions, coagulation disorders, peritonitis, and multi-system organ dysfunction. The initial failure to detect the Pathogen hampered effective therapeutic interventions, resulting in a swift decline in the Patient's clinical status. Tragically, the patient succumbed to the infection before the culture results could be obtained, underscoring the urgency for enhanced diagnostic approaches in cases of suspected TM infections.
Talaromyces marneffei primarily affects severely immunocompromised individuals, notably those with advanced AIDS (3). It is characterized by high rates of misdiagnosis and mortality, reaching as high as 30% even after Antifungal Therapy (4, 5). The patient in this Case had previously been diagnosed with immune thrombocytopenia and had undergone Glucocorticoid therapy for six months, resulting in a relatively immunocompromised state. Talaromyces marneffei is an endemic Pathogen predominantly found in Southeast Asia and southern China (6, 7). In regions where the Disease is prevalent, its occurrence among hospitalized AIDS patients ranges from 4% to 16% (3, 8-10).
Talaromyces marneffei, initially described as Penicillium marneffei (PM), was reclassified in 2011 to the genus Talaromyces following a detailed molecular analysis (11). Talaromyces marneffei is a thermally biphasic fungal species, exhibiting a Mycelial Phase when cultured at 25℃ and a yeast phase when cultured at 37℃, with the latter being the main pathogenic phase. The mycelial-phase conidia of TM are airborne and can be inhaled into the respiratory tract of a Host. In hosts with normal Immune Function, these fungal cells are destroyed by various immune cells, including macrophages. However, in hosts with poor immune function, the conidia undergo mitotic transformation into the yeast phase and can parasitize the Host. Talaromyces marneffei predominantly targets the monocyte-macrophage reticuloendothelial system. As a result, it is prone to infect and induce disease in tissues and organs within this system, including the lymph nodes, lungs, liver, and spleen. in the yeast phase, TM shows increased expression of various Proteins and Enzymes, including Catalase, Isocitrate Lyase, Hsp90, binding proteins, and cytochrome P-450, which may also be associated with its pathogenicity (12). Definitive diagnosis of TM relies on a positive pathogen culture, which typically requires 3 - 14 days. Thus, early diagnosis is challenging and often delayed, especially in patients who do not present with a rash. This delay can lead to increased morbidity and mortality rates (13).
Talaromyces marneffei infections can proliferate across multiple organs and systems. In the Skin and Mucous Membranes, various Rash manifestations such as papules, Nodules, and Ulcers can occur. The Respiratory System, encompassing the upper respiratory tract, lower respiratory tract, and tracheobronchus, may also be involved, potentially leading to cervical lymph node enlargement. Additionally, enlargement of abdominal lymph nodes may occur, with extensive distribution in the abdominal cavity and retroperitoneal area, especially around the mesenteric branch blood vessels and mesenteric root (14). In this case, the patient did not present with a skin rash, ulcer formation, or superficial lymph node enlargement, but significant deep lymph node enlargement was noted, especially in the abdomen. This pattern of lymph node enlargement aligns with those reported in the literature. However, due to the patient’s inability to undergo an abdominal lymph node biopsy, the precise nature of the enlarged lymph nodes remained undefined. Involvement of the digestive system can also present with non-specific symptoms like bloating, abdominal pain, diarrhea, and even hepatomegaly, with elevated aminotransferase and mild to moderate elevation of bilirubin. Pathologically, TM-induced liver disease can be divided into diffuse, granulomatous, and mixed types. Patients with the granulomatous type generally exhibit better cellular immune function, while those with the diffuse type exhibit poorer cellular immune function, with the mixed type falling between the two (15). However, Liver Failure as a primary manifestation of liver lesions in TM infection is rare and currently undocumented in existing literature. Here, the patient presented with liver failure upon admission. Rigorous investigations were undertaken to ascertain the cause of the condition, systematically ruling out various factors including immune-related causes, drug-induced effects, alcohol-related issues, and infections by both hepatotropic and non-hepatotropic viruses. The eventual positive culture from pleural and ascitic fluid samples confirmed TM infection. Thus, we speculate that the liver failure in this patient may be associated with invasion of the mononuclear macrophage reticuloendothelial system in the liver.
Acute liver failure (ALF), a critical condition marked by rapid Liver Impairment and potential reversibility, often affects individuals without previous liver disease. It is characterized by liver injury, coagulopathy (international normalized ratio ≥ 1.5), and Hepatic encephalopathy, and can lead to severe clinical deterioration (16). Furthermore, its etiologies are varied, leading to a wide range of clinical presentations that can affect virtually every organ system (17). The 2023 American Gastroenterological Association guidelines for Acute liver failure identify several causes of ALF, including acetaminophen hepatotoxicity, idiosyncratic drug-induced liver injury, viral Hepatitis, pregnancy-related, autoimmune hepatitis, wilson disease, mushroom poisoning, budd-chiari syndrome, ischemic liver injury, and malignant infiltration (18).
In this Patient, considerable efforts were made to identify the cause of his ALF, with consideration of these guidelines. However, investigations revealed no evidence of drug or alcohol use, immunization reactions, viral infections, ischemic conditions, pregnancy, mycotoxicosis, or hepatic malignancy. Notably, while fungal infections are not listed as a typical cause of ALF in the guidelines, the detection of TM in both pleural and ascitic fluid cultures was suggestive as a potential cause of liver failure. Talaromyces marneffei infection can affect multiple or single organ systems, including the kidney, coagulation, hematologic, digestive, and respiratory systems. In this case, liver lesions were the most significant and rapidly progressed to liver failure after disease onset. Despite prompt and aggressive hepatoprotective and liver replacement therapy after admission, the patient’s condition deteriorated rapidly, primarily due to the initial lack of clarity regarding the cause of liver failure and the absence of targeted etiological treatment.
Thus, we propose that TM infections may also be a causative factor of ALF. Unfortunately, due to poor coagulation, Liver puncture for a definitive diagnosis was not performed on this Patient. Future studies should include larger cohorts and employ longitudinal study designs to better understand the progression from TM infection to ALF and other organ failures.
The early diagnosis and treatment of TM infections present considerable challenges, primarily due to the extended culture period (up to two weeks) required for its identification. However, recent advancements in diagnostic techniques, particularly the increasing availability and use of gene sequencing of pathogenic microorganisms in clinical settings, offer promising alternatives. Talaromyces marneffei infections are notably prevalent in immunocompromised populations, those with long-term use of glucocorticoids or immunosuppressants, and individuals with Diabetes Mellitus. Therefore, in patients with ALF and a history indicative of these risk factors, the possibility of TM infection should be considered. Proactive and thorough investigations for pathogenic evidence are crucial to prevent diagnostic and treatment delays. Given the prolonged culture period required for TM, metagenomic next-generation sequencing could serve as a more rapid method for identifying pathogens. Additionally, PET-CT scanning can be utilized to identify highly metabolized lymph nodes or bone marrow, which are characteristic of TM infection, allowing for targeted biopsies to further investigate pathogenic evidence. While the early diagnosis may not mitigate the potential adverse effects of treatments like amphotericin B in patients with compromised liver function, as was the case in this patient, prompt recognition and treatment of TM infections are vital to reducing mortality. Early and accurate diagnosis, followed by appropriate treatment, is essential to improve outcomes in such patients.