1. Introduction
Acquired immunodeficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV), which attacks the immune system once it infects the human body (1). Hepatitis B virus (HBV) infection is the primary cause of chronic viral hepatitis, cirrhosis, hepatocellular carcinoma (HCC), and acute/chronic/subacute liver failure in China (2). The HIV infection markedly accelerates HBV-related liver disease progression through impaired immune control (3). It also influences the choice of combined antiretroviral therapy (cART) protocols, impacting patient survival and prognosis. With the promotion of cART, opportunistic infections and mortality rates in HIV/AIDS patients are gradually decreasing, leading to improved long-term prognosis and lifespan (4). Patients co-infected with HIV/HBV undergoing standardized cART can achieve high rates of HBV-DNA inhibition, with some experiencing serological responses. End-stage liver disease has become a significant cause of morbidity and mortality in HIV patients with HBV infection. Compared to HBV single infection, especially without antiviral treatment, HIV-HBV co-infected patients experience faster progression of liver fibrosis and have a higher risk of end-stage liver disease, including cirrhosis complications, HCC, and HBV-acute-on-chronic liver failure (ACLF). With the promotion of cART, opportunistic infections and mortality rates in HIV/AIDS patients are gradually decreasing, leading to improved long-term prognosis and lifespan. This paper presents the treatment process of a case of AIDS with HBV-ACLF.
2. Case Presentation
2.1. Clinical History
The patient, Mr. Hu, a male and unmarried, was admitted to our hospital on August 16th, 2023, due to "hematemesis and impaired consciousness for 5 days". Shortly before this admission, the patient had experienced sudden hematemesis followed by impaired consciousness and underwent emergency endoscopic variceal ligation at a local hospital. The patient was a healthcare worker with no history of intravenous drug use, occupational HIV exposure, chronic alcohol consumption, or surgical procedures. Thirty years prior, the patient tested positive for HBsAg during a routine physical examination but did not receive anti-HBV treatment. Six years ago, he was diagnosed with HIV infection and began antiretroviral therapy (ART) with lamivudine (3TC), TDF, and LPV/r. Two years ago, the patient voluntarily discontinued ART. In June 2023, he resumed antiviral therapy with bictegravir (BIC)/emtricitabine (FTC)/tenofovir alafenamide (TAF).
2.2. Physical Examination
Upon this admission, a comprehensive physical examination of the patient revealed: Confusion, presence of liver palms and spider angiomas, jaundice of the skin and sclera, pale conjunctiva, normal cardiopulmonary auscultation, abdominal distension with soft musculature, no tenderness or rebound tenderness, no hepatosplenomegaly on palpation, tympanic percussion note over the abdomen, positive shifting dullness, normal bowel sounds, and mild edema of both lower limbs.
2.3. Laboratory/Imaging Findings
1. Complete blood count: The WBC 3.9 × 109/L, Hb 91 g/L, PLT 70 × 109/L, INR 2.49.
2. Blood biochemistry: The TBIL 185.6 μmol/L, DBIL 99.7 μmol/L, ALT 378 U/L, AST 581 U/L.
3. Renal function: Normal.
4. Ascites analysis: Yellow, no clots, Rivalta test (-), cell count 0.21 × 109/L.
5. Hepatitis B serology: HBsAg (+), HBeAg (+), HBcAb (+); negative for hepatitis A, C, and E antibodies.
6. CD4+ count: 144 cells/μL.
7. AFP: 8.3 ng/mL.
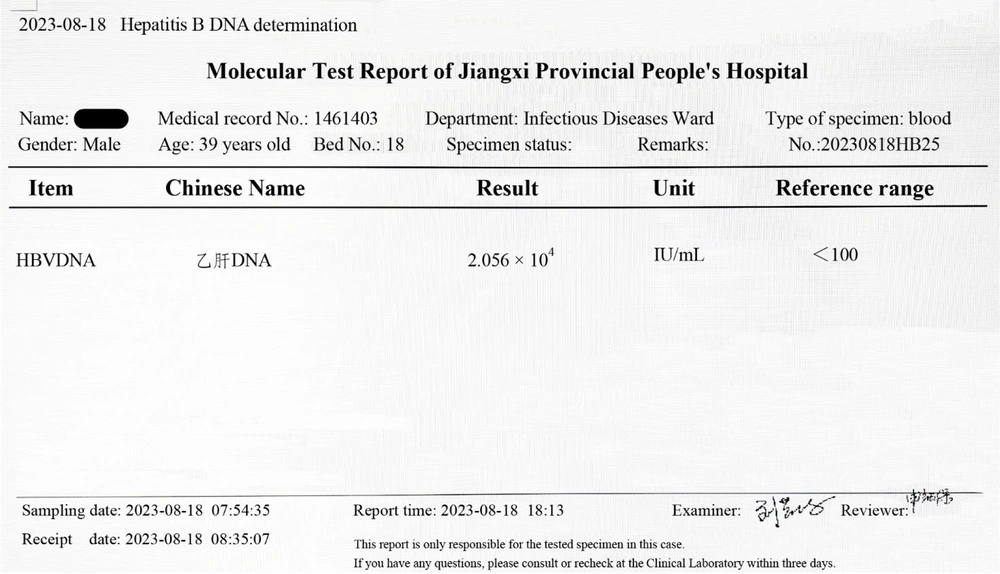
8. The HBV-DNA: 2.056 × 104 IU/mL (Figure 1).
9. PCT: 0.52 ng/mL.
10. The HIV-RNA: Undetectable.
11. Blood ammonia: 56.4 μmol/L.
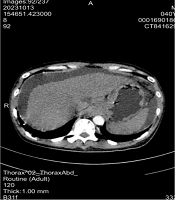
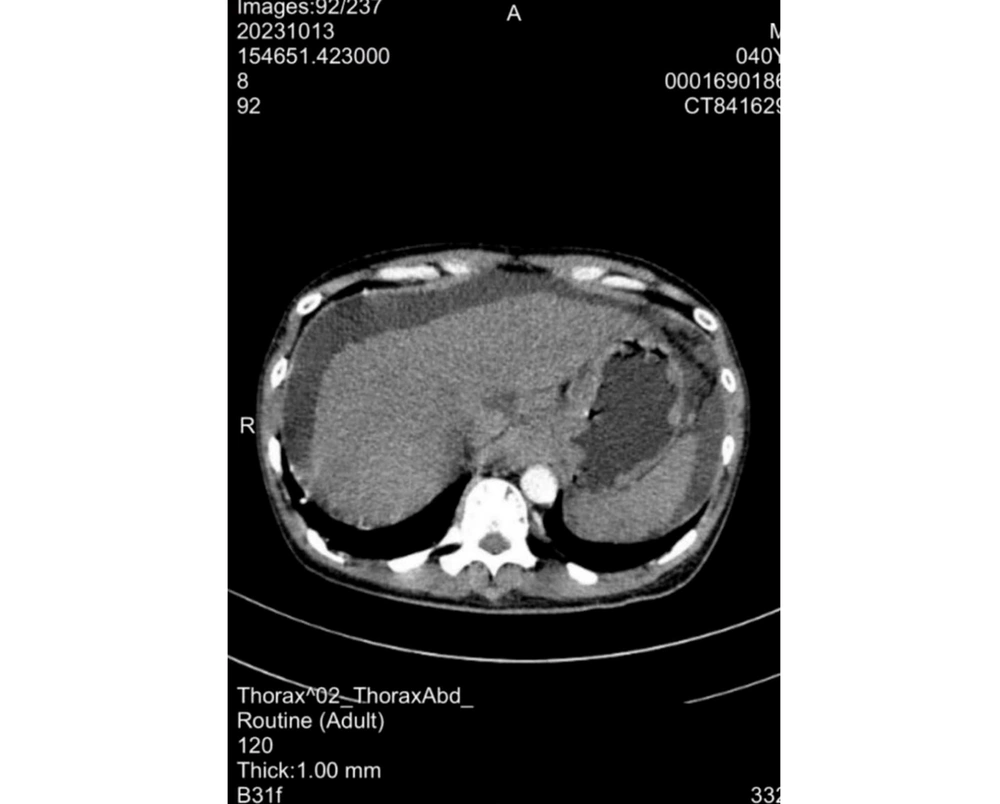
12. Abdominal ultrasound: Massive ascites; continuous drainage via indwelling peritoneal catheter was initiated. Upper abdominal CT: Cirrhosis, ascites, splenomegaly (Figure 2).
Final diagnoses were as follows: (1) The ACLF; (2) AIDS; (3) hepatic encephalopathy (stage 2); (4) esophagogastric variceal bleeding due to cirrhosis; (5) decompensated hepatitis B cirrhosis; (6) spontaneous bacterial peritonitis.
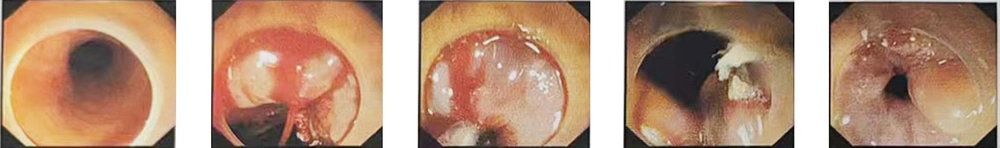
After receiving comprehensive treatment including antiviral therapy, hepatoprotection, anti-hepatic coma measures, anti-infection therapy, albumin and fresh frozen plasma transfusion, the patient’s consciousness gradually improved, and clinical symptoms alleviated. However, on September 1st, he experienced sudden upper gastrointestinal bleeding (~1000 mL). Initial medical therapy was ineffective, prompting endoscopic hemostasis (Figure 3). Post-procedure, hepatic encephalopathy developed but resolved with treatment. No further bleeding occurred, and two consecutive fecal occult blood tests (3 days apart) were negative. Subsequently, oral leukoplakia and repeated detection of fungi in stool led to a diagnosis of gastrointestinal fungal infection, which was treated with oral fluconazole. After three weeks, leukoplakia resolved, stool fungal tests turned negative, and the infection was controlled. Liver function gradually improved, with prothrombin time activity (PTA) stabilizing at 20 - 30%. Ascites and intra-abdominal infection persisted on follow-up ultrasounds. The trend changes in important laboratory data results of the patient during hospitalization before October 6th are shown in Table 1. On October 6th, the patient suffered massive upper gastrointestinal bleeding (> 2000 mL) and died after an unsuccessful emergency transjugular intrahepatic portosystemic shunt (TIPS).
| Variables | 2023.08.16 | 2023.08.21 | 2023.08.26 | 2023.09.01 | 2023.09.12 | 2023.09.18 | 2023.09.23 | 2023.09.28 | 2023.10.05 | 2023.10.16 | 2023.10.26 | 2023.11.05 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBIL (μmol/L) | 185.6 | 266.8 | 409.6 | 322.4 | 277.9 | 239.6 | 216.6 | 196.8 | 196.3 | 201.1 | 206.1 | 149.0 |
| DBIL (μmol/L) | 99.7 | 148.3 | 224.7 | 200.4 | 158.2 | 148.0 | 140.3 | 136.8 | 134.5 | 144.5 | 126.3 | 96.0 |
| Alb (g/L) | 28.2 | 31.6 | 30.3 | 24.8 | 31.8 | 31.1 | 27.2 | 29.0 | 34.1 | 29.4 | 33.4 | 20.8 |
| ALT (U/L) | 378 | 164 | 131 | 118 | 78 | 72 | 57 | 43 | 39 | 37 | 33 | 28 |
| AST (U/L) | 581 | 181 | 212 | 239 | 171 | 168 | 139 | 105 | 103 | 96 | 86 | 77 |
| CHE (IU/L) | 3389 | 2861 | 3082 | 2174 | 2933 | 2750 | 2530 | 1982 | 2332 | 2093 | 1995 | 1146 |
| PT (s) | 33.1 | 28.4 | 26.9 | 35.7 | 29.2 | 29.5 | 27.6 | 28.6 | 25.2 | 28.4 | 28.1 | 180.0 |
| PTA (%) | 16.2 | 23.7 | 25.6 | 20.6 | 27.2 | 22.6 | 29.4 | 23.5 | 28.0 | 23.7 | 28.7 | -- |
| WBC (109/L) | 3.9 | 2.93 | 4.15 | 6.27 | 2.63 | 2.7 | 2.97 | 2.97 | 3.05 | 3.84 | 3.05 | 5.96 |
| N (109/L) | 2.8 | 1.75 | 2.91 | 3.82 | 1.34 | 1.48 | 1.79 | 1.67 | 1.81 | 2.54 | 1.93 | 3.39 |
| Hb (g/L) | 91 | 97 | 113 | 79 | 81 | 90 | 86 | 78 | 79 | 74 | 68 | 56 |
| PLT (109/L) | 70 | 85 | 73 | 85 | 55 | 53 | 60 | 46 | 59 | 56 | 62 | 61 |
| AFP (ng/mL) | 8.3 | 8.9 | 9.1 | 8.7 | 9.4 | 10.5 | 10.6 | 8.7 | 7.9 | 7.3 | 5.2 | 6.8 |
Abbreviation: PTA, prothrombin time activity.
3. Discussion
The HIV-1 and HBV impose a significant global burden. Both viruses can result in chronic disease, cancer, and mortality, with current therapies failing to achieve eradication. Among people with HIV (PWH) globally, up to 20% have HBV co-infection, while a larger proportion has either prior infection (i.e., HBcAb) or is at risk of exposure (5). It is estimated that 8 - 10% of individuals with HIV have chronic HBV infection, with regional variations in co-infection prevalence. Resistance to antiviral drugs frequently develops after prolonged treatment, leading to a loss of clinical efficacy. Co-infection with these viruses exacerbates the negative outcomes. Worldwide, HBV remains the primary cause of chronic liver disease and a leading cause of death (6). The AIDS, caused by HIV infection, has an estimated HIV/HBV co-infection rate of 10% in China. Patients co-infected with HIV and HBV experience accelerated liver disease progression and elevated liver-associated mortality. While cART that includes anti-HBV agents has been successful in suppressing HIV and HBV replication, morbidity and mortality rates remain considerably higher in co-infected individuals compared to those with HIV alone. Co-infection with HIV/HBV can worsen liver inflammation and damage, with a liver-related mortality rate 8 - 19 times (7) higher than that of individuals infected with either HIV or HBV alone. Chronic HBV infection is associated with significant morbidity and mortality, and the presence of coexisting HIV infection can accelerate disease progression. Three French studies (8-10) have shown faster progression to cirrhosis in HIV-co-infected individuals, and a higher risk of decompensation in co-infected individuals with cirrhosis. While our single case cannot establish causality, it contributes to the growing evidence of HIV/HBV synergy in liver disease progression. Larger prospective cohorts are needed to quantify this risk and optimize management strategies.
The presence of HBeAg, a marker of active viral replication, significantly increases the risk of progressive disease, cirrhosis, and eventually, HCC. In patients co-infected with HIV-HBV, the viral loads (VLs) of both HIV and HBV are elevated, liver function damage is more severe than in individuals infected only with HIV, and the risk of developing liver failure is significantly increased (11). When both viruses infect the same cell, HBV gene products can enhance HIV reverse transcription levels, accelerate HIV replication, and result in the depletion of CD4+ T lymphocytes in individuals with HIV infection, altering cytokine levels in the liver environment, thus speeding up the development of liver fibrosis (12). Therefore, early identification and antiviral treatment after HBV/HIV co-infection are critical for disease control. Among ART antiviral medications, there are drugs that inhibit HBV, such as nucleos(t)ide analogues (NAs). When patients discontinue ART drugs [including tenofovir disoproxil fumarate (TDF)] independently, rapid HBV proliferation occurs in the body, and effector T lymphocytes respond strongly. The HBV-DNA levels are generally higher in individuals living with HIV, increasing the risk of progression to end-stage liver disease, cirrhosis, and HCC. Furthermore, HBV reactivation is more common in HIV-positive individuals, particularly in relation to CD4+ T lymphocyte counts (13). This reactivation results in a severe immune response, leading to extensive liver cell necrosis and HBV-ACLF. In such cases, chronic hepatitis B can progress to cirrhosis rapidly after discontinuation of ART. Initially, clinical symptoms such as general fatigue and lassitude may be mild and non-specific to liver failure. However, with recurrence, signs such as multiple ecchymosis, petechiae, worsening jaundice, hypoalbuminemia, persistent abdominal distention, increasing peritoneal effusion, spontaneous peritonitis, and endotoxemia all indicate HBV-ACLF.
The ACLF represents a severe form of acute decompensation (AD) in liver cirrhosis, with a 28-day mortality rate of ≥ 20% compared to ≤ 5% in AD patients with cirrhosis without ACLF. The ACLF is characterized by the failure of one or more of the six major organs or systems: Liver, kidneys, brain, coagulation, circulation, and respiration, along with systemic inflammation triggered by acute events (intrahepatic and/or extrahepatic injury). Currently, liver transplantation (LT) remains the only definitive treatment to reduce ACLF mortality (14), though its availability is limited by a shortage of donor organs and high mortality rates among those on waiting lists. In China, HBV continues to be the primary cause of ACLF. In addition to host factors such as age, gender, and HBV-DNA quantification, other risk factors for HBV reactivation among PWH include low CD4 counts, elevated HIV VL, and withdrawal of HBV-active agents (15). The HBV-ACLF progresses rapidly, carries a high mortality rate, and has a poor prognosis, making it one of the prevalent end-stage liver diseases in clinical settings (16). Principal symptoms include jaundice, coagulation dysfunction, hepatorenal syndrome, hepatic encephalopathy, and ascites (17). The rate of bacterial infection in patients with liver cirrhosis is about 5 times higher than in the general population (18). The mortality rate for ACLF patients with complications is significantly higher than for those without complications, with mortality rates at 28 or 90 days being 4 times and 8 times higher, respectively (19). During the disease course, patients may develop gastrointestinal fungal infections. In the decompensated phase of liver cirrhosis, both congenital and adaptive immune failures can activate intracellular signaling pathways and damage gastrointestinal lymphoid tissue, and circulating immune cells. This condition, known as cirrhosis-related immune dysfunction syndrome, is considered a factor contributing to the increased incidence of systemic fungal infections in patients with cirrhosis (20). Currently, some researchers (21) have examined the relationship between CD4+ T cells and opportunistic infections, noting that a rapid decline in CD4+ cell levels often signals a heightened risk of opportunistic infections. However, this patient did not experience any additional opportunistic infections throughout the disease course, such as tuberculosis or PCP infection; this may be associated with the quantitative negative conversion of HIV-RNA in this patient, though the exact reason requires further investigation.
The HBV is primarily hepatophilic, but HIV is also lymphophilic, so in co-infected patients, HIV and HBV coexist at the cellular level. This finding shows the complex interaction between HIV and HBV. The HBV replication values were higher when HIV infection co-existed. This may be caused by either direct HIV induction or the decreased immune control of HBV infection as a result of HIV-induced cellular immunodeficiency (3). Several studies have demonstrated that various cell types in the liver are permissive to HIV infection in vitro, including hepatic stellate cells (HSCs), Kupffer cells, and hepatocytes. The persistence of HIV infection in liver tissue after the initiation of ART in PWH remains uncertain. However, research involving animal models, such as SIV-infected macaques and HIV-infected humanized mouse models, indicates that HIV can persist in the liver during ART, primarily within Kupffer cells. In the absence of active viral replication on ART, HIV may still contribute to liver inflammation and fibrosis through the binding of gp120 to CXCR4, which is expressed on hepatocytes and HSC (22). The impact of HIV infection and its proteins in the liver has primarily been studied in the context of HIV-HCV co-infection in vitro, with less focus on HIV-HBV co-infection. The HIV infection, whether alone or in the presence of HCV, has been shown to induce profibrotic processes in hepatocyte and HSC cell lines, including increased chemokine production, HSC migration, hepatocyte apoptosis, and expression of profibrotic genes (23). The immunopathogenesis of ACLF in this case likely involved synergistic mechanisms (24): (1) The HIV-induced CD4+ depletion impairs HBV-specific immune control, leading to unchecked viral replication and direct hepatocyte injury; (2) HAART-associated immune restoration (IRIS) may trigger excessive inflammation against HBV antigens, mimicking fulminant hepatitis. This aligns with reports of HBV flare after chemotherapy withdrawal. A previous study by Colin et al. (8) suggests that HIV/HBV co-infection accelerates liver disease progression.
Among PWH with chronic HBV infection, lifelong HBV treatment is recommended, which may include TAF/TDF and 3TC/FTC or entecavir (ETV) in combination with a complete HIV regimen (25). Treatment objectives are similar to those for chronic HBV monoinfection, including HBV suppression, HBsAg seroconversion, and preventive measures such as HCC screening. In this case, the patient opted for BIC as part of the antiviral therapy for ART treatment. The BIC is the smallest triple compound single-tablet anti-HIV virus preparation based on integrase chain transfer inhibitors, consisting of BIC, FTC, and TAF. In the study by Yun et al. (26), BIC tablets were the primary suspected cause in 844 adverse event reports. The proportion involving the liver and gallbladder system was 1.77% (21 cases). Currently, there are limited studies on the drug’s impact on the liver and on the prognosis of ACLF both domestically and internationally. Although approved for use in patients with mild/moderate liver injury, the causes of ACLF in this case primarily involve a rapid progression of hepatitis B to cirrhosis following ART discontinuation, with liver function damage occurring on a cirrhotic basis, induced by HBV. Concurrently, in the BIC formulation, FTC and TAF can effectively inhibit HBV, which are key drugs in controlling HBV DNA; TAF has been shown to significantly improve conditions in patients with chronic hepatitis B (27). Several ART strategies could be considered for similar HIV/HBV co-infected patients presenting with ACLF. When our patients were treated with BIC/FTC/TAF, the renal safety was high considering that TAF may have higher intracellular drug levels; compared with alternatives containing TDF, it may be beneficial for patients with renal impairment (28). Importantly, any ART regimen for co-infected patients must include dual-active agents to suppress both viruses. In conclusion, although the HIV virus is not inherently hepatotropic and does not cause liver cell damage, co-infection with HIV/HBV can accelerate the progression from hepatitis to cirrhosis, resulting in a considerably higher mortality rate among these patients compared to those with chronic hepatitis B alone. This gap underscores the need for innovative therapeutic strategies that can more effectively address the complex interplay between HIV and HBV. Emerging therapeutic advancements presented at CROI 2023 offer transformative potential for managing HIV and viral hepatitis (29). Therefore, further research is essential to elucidate the specific relationship between the two.