1. Background
The Hepatitis B virus (HBV) genome comprises a partially double stranded 3.2 kb DNA with four open-reading frames (ORF). These ORF’s encode the polymerase gene (P gene), core antigen (C gene), large, medium and small surface antigen proteins (S gene) and the X protein (X gene) (1). The full genome analysis of HBV revealed abundant information including identification of mutations reported worldwide in all the four open reading frames (2). Malaysia is a country of medium seroprevalence for the HBV surface antigen (HBsAg) in the general population (range 1.5–9.8%) (3). Most carriers are infected prenatally owing to the high viral load in Malaysian women of child-bearing age (4). The S gene region of the hepatitis B virus is of particular interest, since it is responsible for the expression of surface antigens and classification of HBV strains. Initially, HBV strains were classified based on the immunological heterogenecity of HBsAg. Following the advances in molecular techniques, serotype-based classification was established based on the variants in the major hydrophilic region (MHR) (5). The presence of immune escape mutants in this region gives HBV variants a distinct survival advantage, permitting the mutant virus to escape from the immune system. This is worrisome in the context of public health, because these mutant viruses can reduce sensitivity of diagnostic assay by causing false negative result. Besides, it can result in failure of vaccination if not detected early.
2. Objectives
The current study aimed to identify the occurrence of S gene mutants among Hepatitis B carriers in Malaysia.
3. Materials and Methods
3.1. Ethics Statement
The current study was ethically approved by Ethics and Medical Research Committee, Ministry of Health Malaysia (Reference number: NMRR-12-311-11789). The ethics committee did not deemthe patient consent necessary,since the samples used were retrospective and confirmed as Hepatitis B carriers by Selayang Hospital, Kuala Lumpur, Malaysia.
3.2. Serum Samples
A total of 93 retrospective blood serum samples of the confirmed Hepatitis B carriers were obtained in a volume of 2 mL from the Pathology Unit, Selayang Hospital, Kuala Lumpur, Malaysia. Hepatitis B carriers are defined as persons positive for Hepatitis B surface antigen (HBsAg) for more than six months. The samples were randomly collected regardless of age, race, sex, treatment, and symptoms for a period of three years (2012-2014). The demographic data of the patients participating in the study were summarized in Table 1.
| Group 1, Hbe Ag Positive (n = 24) | Group 2, Hbe Ag negative (n = 69) | |
|---|---|---|
| Age, y | 45.6 ± 15.2 | 50.5 ± 15.8 |
| Sex | ||
| Female | 14 | 43 |
| Male | 10 | 26 |
| HBV DNA copy, IU/mL | 5.2 × 107 ± 1.3 × 108 | 1.5107 ± 6.3 × 107 |
| Normal ALT level, % | 79.2 | 91.3 |
| Elevated ALT level, % | 20.8 | 8.7 |
| No. HCC | 6 (25.0) | 7 (10.1) |
| Liver cirrhosis | 5 (20.8) | 16 (23.2) |
| Chronic Hepatitis B | 18 (75) | 41 (59.4) |
| Fulminant Hepatitis B | 0 (0) | 1 (1.4) |
| Acute Hepatitis B Flare | 1 (4.1) | 2 (2.9) |
| Asymptomatic | 0 (0) | 2 (2.9) |
Demographic Data of Patients Participatingin the Study a
3.3. HBV DNA Extraction
HBV DNA was extracted from sera samples using High Pure Viral Nucleic Acid Extraction Kit (Roche, USA) according to the manufacturer’s instructions. The final elution contained 50µL of viral DNA and was stored at -20ºC for long term usage.
3.4. HBV Full Genome Amplification
The 3.215 kb length HBV full genome was amplified using 14 sets of the previously published oligonucleotides (6, 7). The primers published by Sugauchi et al. (6) were used to perform the nested PCR whereas amplification with primers published by Kahila Bar-Gal et al. (7) were done by direct PCR. All oligonucleotides used in the study are listed in Table 2. A fragment of 3.2 kb was amplified in the first round PCR using HB8F and HB6R primers. A second round of PCR using 11 sets of oligonucleotides (HB1F-1R to HB12F-12R) was performed on the 3.2 kb fragment to produce 11 overlapping fragments that contributed to full genome sequence when aligned. Due to possibility of gaps, three alternate primer sets (HEP 13F-13R, HEP14F-14R and HEP15F-15R) were also used. All amplification reactions were carried out in a 96-well Thermal Cycler (Bio Rad, USA). The first round of PCR was undertaken for 35 cycles (94°C for 1 minute, 55°C for one minute and 72°C for 1.5 minutes) followed by an extension reaction at 72°C for five minutes. The second round PCR was performed for 30 cycles (94°C for one minute, 55°C for one minute and 72°C for one minute) followed by extension at 72°C for five minutes. The first roundPCR reaction liquidcontained 12.5 uL of 2x MiFi Mix, 1.0 uL of each oligonucleotides (10 uM), 5.5 uL sterile dH2O and 5 uL of the extracted HBV DNA. The second round PCR reaction liquid contained the same reagent concentrations for each of the 11 sets of oligonucleotides, except that only 2 uL of the first round PCR product was used as a template. Amplification with the three alternate primers were performed at 94°C for 10minutes followed by 40 cycles of 94°C for 45 seconds, 56°C for 45 seconds and 72°C for 45 seconds and a final extension of 72°C for 10 minutes. The reagent composition was the same as the first round PCR. A synthetic Hepatitis B virus isolate M1 (GenBank Accession number: GQ924603) was used as the positive control in the initial run to test the primers. A negative non-template control was also included in each run of the PCR.
| Oligonucleotide Identity | Sequence (5’-3’) | Reference |
|---|---|---|
| HB1F | AAGCTCTGCTAGATCCCAGAGT | Sugauchi et al. (2001) |
| HB1R | GAAACATAGAGGTGCCTTGAGCAG | Sugauchi et al. (2001) |
| HB2F | TGCTGCTATGCCTCATCTTC | Sugauchi et al. (2001) |
| HB2R | CATACTTTCCAATCAATAGG | Sugauchi et al. (2001) |
| HB3F | GCCAAGTCTGTACAACATCTTGAG | Sugauchi et al. (2001) |
| HB3R | AGTTGGCGAGAAAGTGAAAGCCTG | Sugauchi et al. (2001) |
| HB4F | CCTATTGATTGGAAAGTATGTCA | Sugauchi et al. (2001) |
| HB4R | CGGGACGTAGACAAAGGACGT | Sugauchi et al. (2001) |
| HB5F | CTCTGCCGATCCATACTGCGGAA | Sugauchi et al. (2001) |
| HB5R | TTAACCTAATCTCCTCCCCCA | Sugauchi et al. (2001) |
| HB6F | TTGTYTACGTCCCGTCGGCG | Sugauchi et al. (2001 |
| HB6R | AACAGACCAATTTATGCCTA | Sugauchi et al. (2001) |
| HB8F | TTCACCTCTGCCTAATCATC | Sugauchi et al. (2001 |
| HB8R | ATAGGGGCATTTGGTGGTCT | Sugauchi et al. (2001) |
| HB9F | TCAGGCAACTATTGTGGTTTCA | Sugauchi et al. (2001) |
| HB9R | GGATAGAACCTAGCAGGCAT | Sugauchi et al. (2001) |
| HB10F | CGCAGAAGATCTCAATCTCGG | Sugauchi et al. (2001) |
| HB10R | GGGTTGAAGTCCCAATCTGGATT | Sugauchi et al. (2001) |
| HB11F | GGGTCACCATATTCTTGGGAA | Sugauchi et al. (2001) |
| HB11R | GAACTGGAGCCACCAGCAGG | Sugauchi et al. (2001) |
| HB12F | GTGGAGCCCTCAGGCTCAGG | Sugauchi et al. (2001) |
| HB12R | CGAGTCTAGACTCTGTGGTA | Sugauchi et al. (2001) |
| HEP13F | GGACTCTTGGACTCTCAGCAA | Kahila Bar-Gal et al. (2012) |
| HEP13R | GGCAGAGGTGAAAAAGTTGC | Kahila Bar-Gal et al. (2012) |
| HEP14F | GCATAAATTGGTCTGTTCACCAG | Kahila Bar-Gal et al. (2012) |
| HEP14R | CTCCACAGAAGCTCCAAATTC | Kahila Bar-Gal et al. (2012) |
| HEP15F | GAATTTGGAGCTTCTGTGGAG | Kahila Bar-Gal et al. (2012) |
| HEP15R | TCATCAACTCACCCCAACACAG | Kahila Bar-Gal et al. (2012) |
The Oligonucleotides Used in the Amplification of HBV Full Genome
3.5. Post PCR Purification and Sequencing
A 15uL aliquot of each PCR reaction was analyzed on 2% agarose by gel electrophoresis and viewed under UV illumination. The agarosewas presented with Red Safe Dye (Intron Biotech, Korea) as an alternative to ethidiumbromide. The corresponding amplicons were extracted from the agarose gel and purified using Gel Extraction Kit (Qiagen, USA) according to the manufacturer’s instruction. The final elution contained 35uL of purified PCR amplicons from which 5 uL was reanalyzed on 2% agarose gel to confirm that the purification steps were performed precisely. All purified PCR amplicons were subjected to cycle sequencing using corresponding sense and antisense oligonucleotides prior to sequencing in 3730 Genetic Analyzer (Applied Biosystem, USA).
3.6. Data Analysis
The overlapping sense and antisense sequences, obtained by sequencing, were aligned to produce a full length genome of HBV using CLUSTAL Omega software (https://www.ebi.ac.uk/Tools/msa/clustalo/). The reference sequence used in the alignment was Hepatitis B virus isolate M1 (GenBank Accession number: GQ924603).The genotypes of Malaysian Hepatitis B carriers were determined by the Genotyper bioinformatics tool in the HEPSEQ: International Repository for Hepatitis B Virus Strain Data by the assembled genome sequences as an input (http://www.hpabioinformatics.org.uk/HepSEQResearch/Public/Tool/genotype_tool.php). Apart from that, phylogenetic trees were constructed to determine the genotypes and sub-genotypes of the full length sequences of Hepatitis B isolates using Mega 6.06, and neighbor joining method (bootstrap replication 1000x). Subsequently, the position of the S gene region was identified and analyzed for the presence of variants. In addition, the obtained sequences were also analyzed at the basal core promoter (BCP), precore (PC), and core (C) region.
4. Results
4.1. HBV Full Genome Amplification and Sequencing
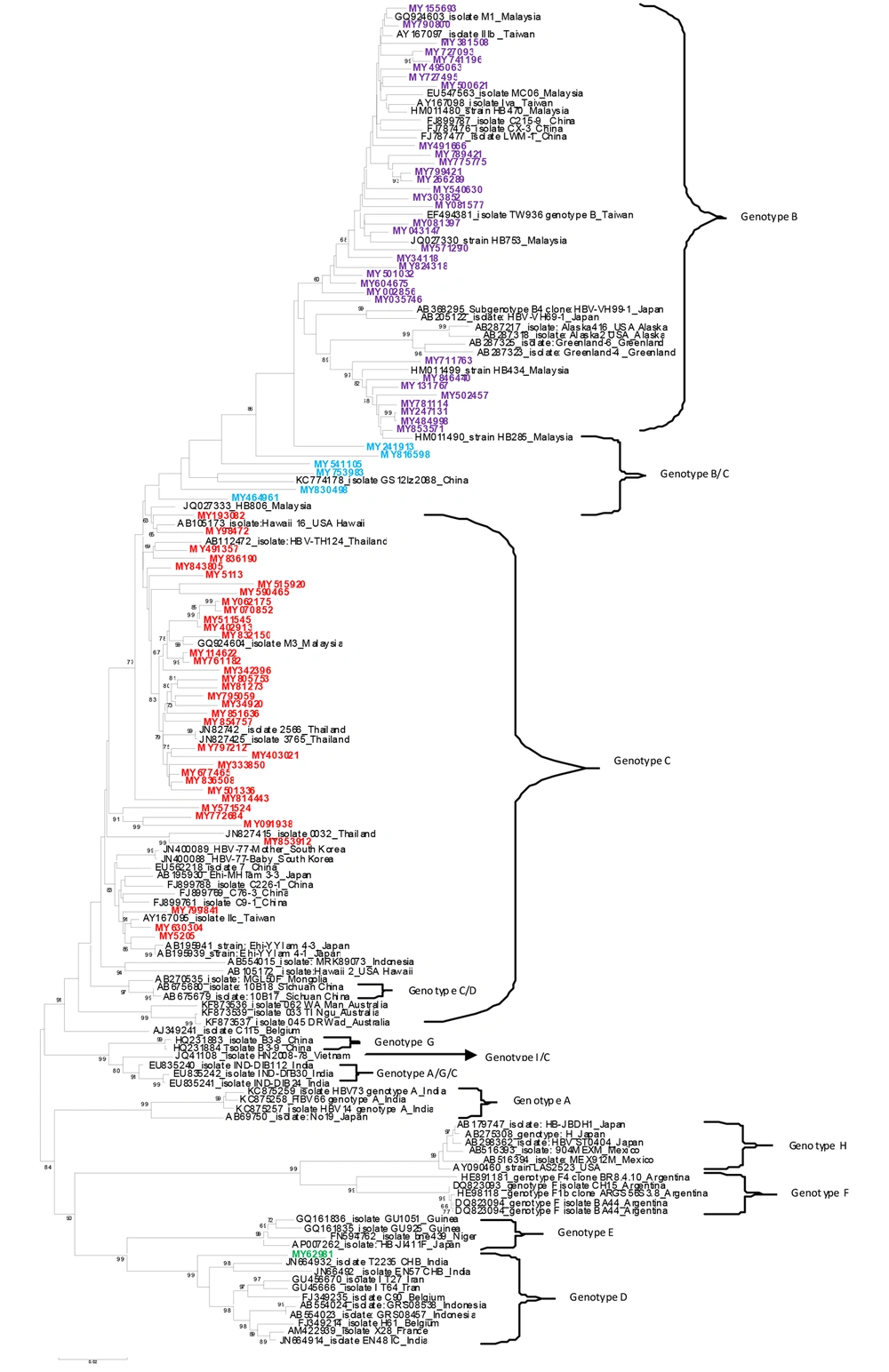
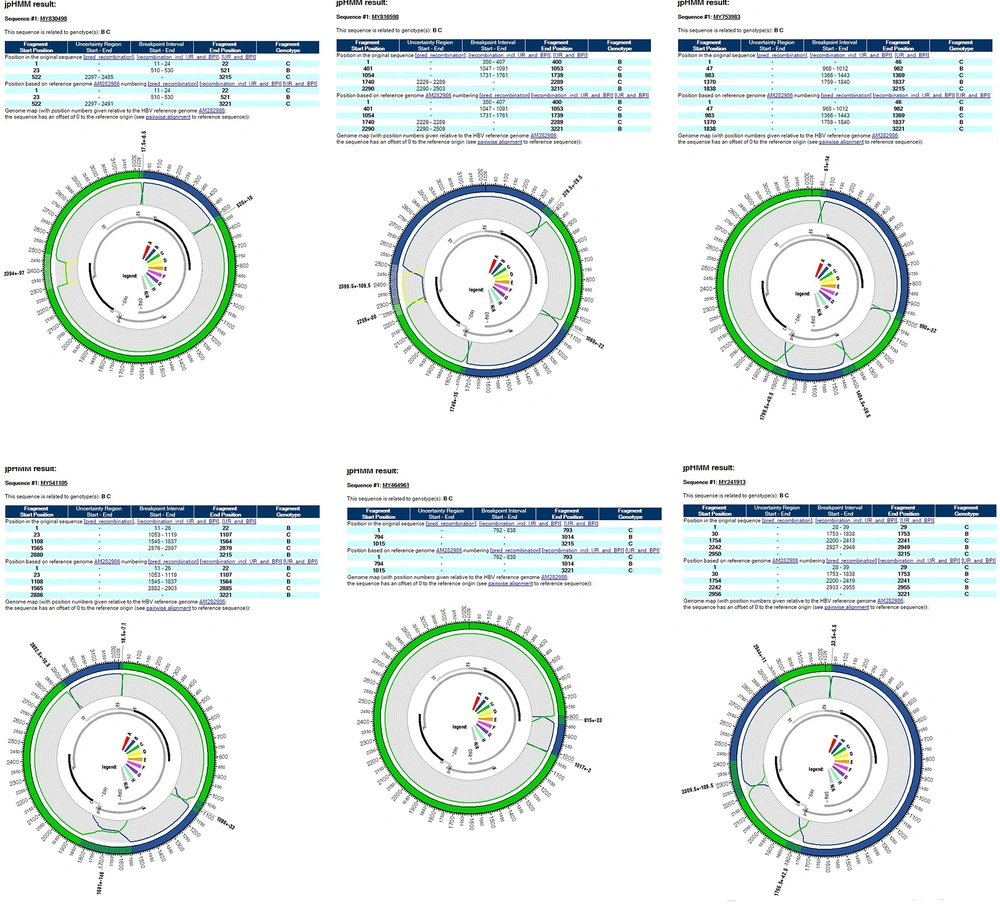
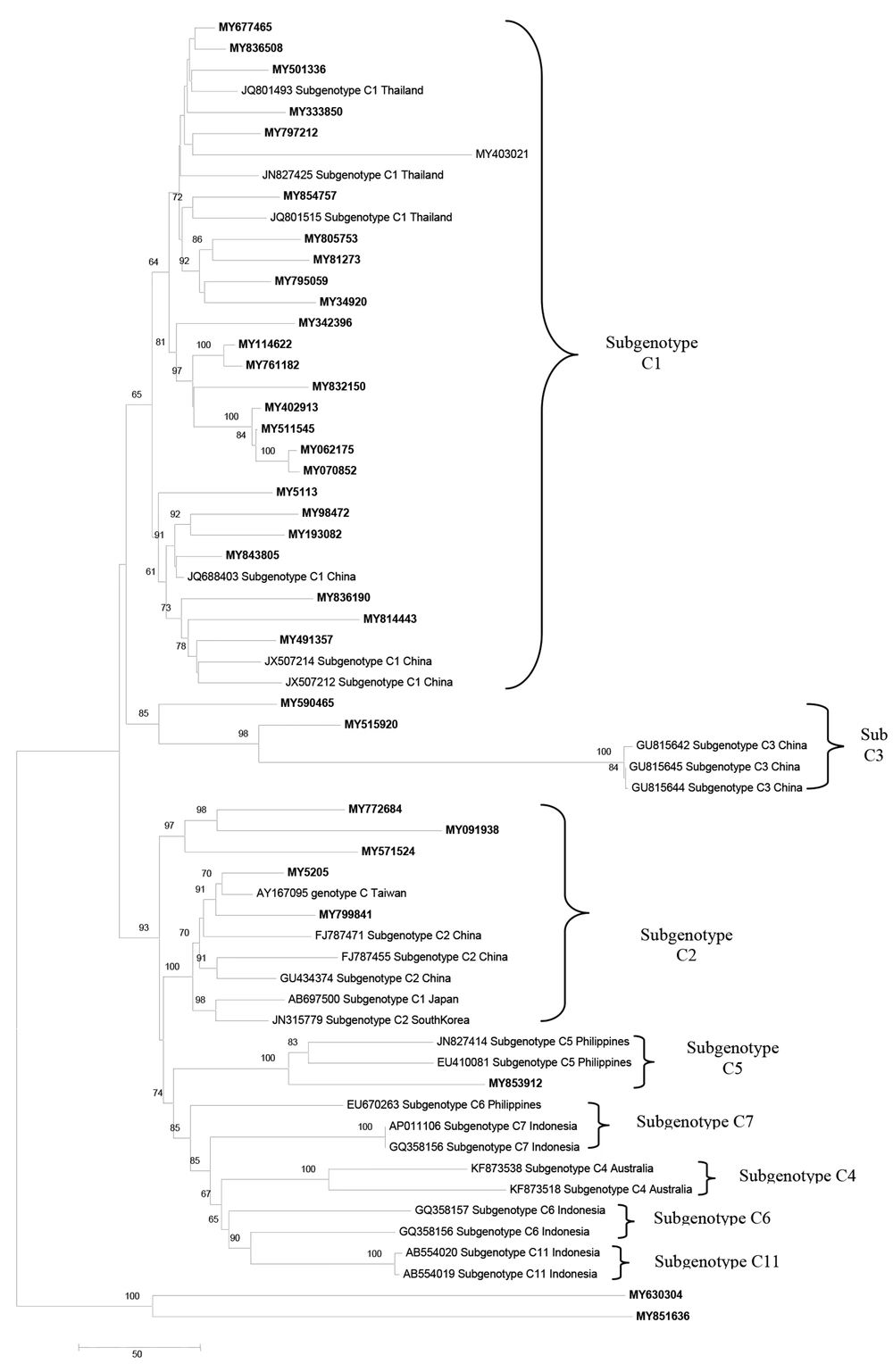
Of the total 93 analyzed samples, 76 HBV complete nucleotide and 17 partial nucleotide sequences were obtained by PCR amplification and sequencing. The sequences were deposited into the GenBank (Accession numbers: KJ803752-KJ803827).Genotyping of the HBV full length and partial sequences based on the HEPSEQ software revealed a distribution of 49.46%, 48.39% and 2.15% of genotypes C,B, and D ,respectively. The phylogenetic tree for 76 full-length isolates revealed similar clusters of genotypes with the exception of six sequences (MY830498, MY816598, MY753983, MY541105, MY241913 and MY464961) which fell into the B/C recombinant cluster (Figure 1). The HEPSEQ software indicated that these sequences were genotype C with low probability, and therefore suggests possible recombinant strains. Jumping profile Hidden Markov Model (jpHMM) bioinformatics tool was utilized to confirm the presence of recombinant further. This software served as a tool to detect recombination in HIV-1 and hepatitis B virus (HBV) genomes (8). All six sequences were confirmed to be B/C recombinant strain (Figure 2). The remaining 70 isolates were also analyzed and no recombinant strains were found.
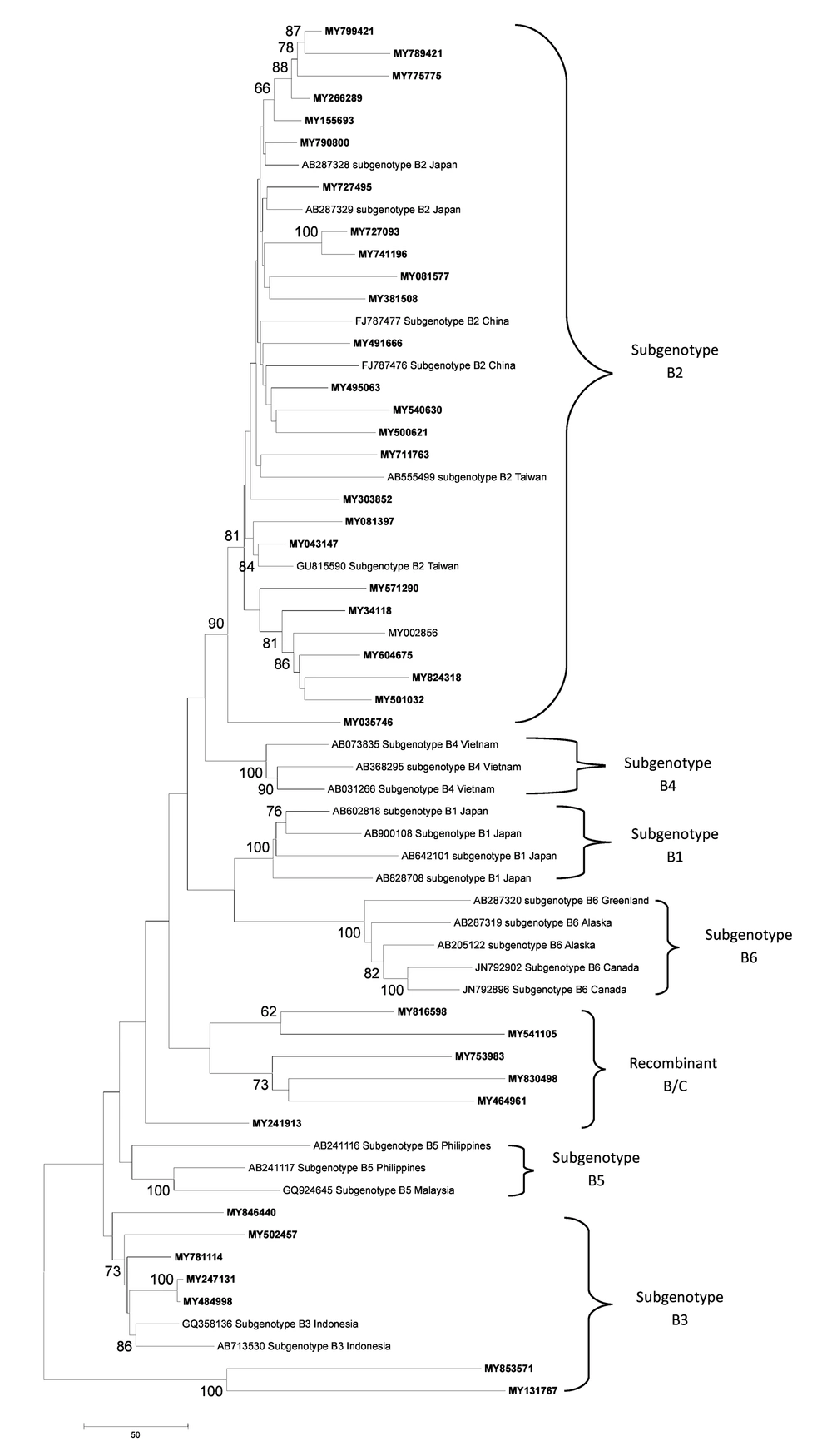
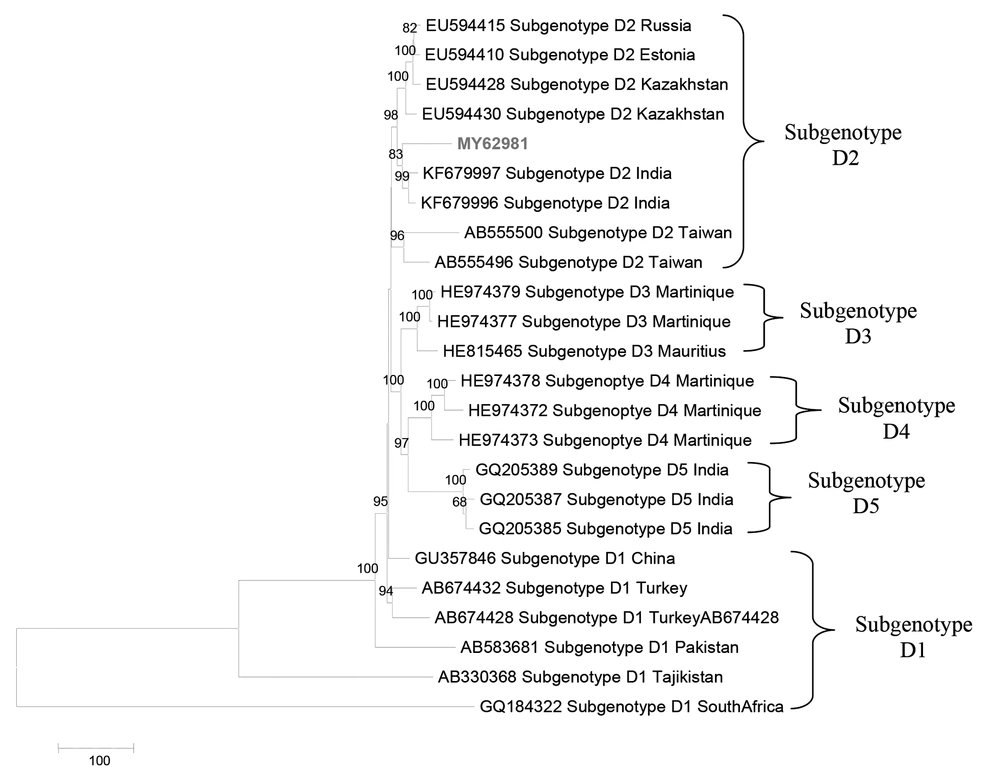
Phylogenetic analysis also demonstrated that Malaysian HBV isolates belonged to sub-genotypes B2 (78.79%) and B3 (21.21%) for genotype B, sub genotype D2 (100%) for genotype D and sub geno type C1 (75.76%), C2 (15.15%), C3 (6.06%) and C5 (3.13%) for genotype C (Figure 3 - 5).
4.2. S Gene Analysis
The S gene region in the whole genome of HBV was identified to be from 2848nucleotide (nt) to 3215nt and joined at position 1nt to 835nt. Two significant S gene mutations were found in the Malaysian HBV sequences. The W182 stop codon (W182*) and deletion at open reading frame (ORF) of pre-S1 were present with the frequencies of 2.2% (2.93) and 5.4% (5.93), respectively. There was no presence of MHR mutation in the S gene region. The position of W182* and pre-S1 deletion were shown in Figure 6.
A) Deletion at ORF of Pre S1 region of the HBV genome in five patients Comparison was made with the reference sequence GQ924603 by alignment in Clustal Omega tool; B) W182 stop codon in the S gene region of HBV in two patients. A substitution of TGG-TGA at nucleotide position 700 in hepatitis B full genome contributed to the stop codon (opal). Comparison was made with reference sequence (GQ924603) and wild type patients.
4.3. Case Report of W182 Stop Codon Mutation
The characteristics of the two patients with W182* mutation were summarized in Table 3. Patients MY541105 and 062175 were both Malaysian Chinese, male, infected with HBV genotype C and diagnosed for chronic hepatitis B. Patient MY541105 didnot receive treatment and was not followed up due to transportation issues. Patient 062175 was given entecavir for a month in 2012. Liver CT revealed large hepatocellular carcinoma (HCC) in the right lobe of the liver and within four months, progression of the disease was observed as the huge HCC was complicated with bleeding and the presence of regenerate nodule in segment three of the liver. Patient has had no recent visits to the hospital.
| Patient ID | Sex | Age | History of Treatment | HBV VIRAL LOAD | HBV Genotype | HBsAg Status | Clinical Diagnosis |
|---|---|---|---|---|---|---|---|
| MY541105 | M | 24 | No treatment recorded | NA | C | Positive | -Chronic Hepatitis B; Mild neutropenia |
| MY062175 | M | 46 | Given entecavir in April 2012, prescribe for 1 month only | -Initial count in April 2012:1.2 × 106 IU/mL; In April 2013:236 IU/mL | C | Positive | -Chronic Hepatitis B; Known Hepatitis B diagnosed at age 23yrers-HBsAg +ve; -HBeAg+ve; -Hepatocellular CA showed large HCC in right lobe that progressed to bleeding; -Some fluid around tumor |
Characteristics of the Patients With W182 Stop Codon Mutation a
4.4. Case Report of ORF Deletion in Pre-S1
Five patients (MY630304, MY62981, MY853571, MY851636 and MY131767) possessed nucleotide deletions at the ORF of pre-S1 region of HBV. The number of nt deletions in this region varied in all five patients; MY630304, MY62981, MY853571 and MY851636, and MY131767demonstrated 21, 33, 12, and 17 nt deletions, respectively. All patients were female except one, with the age ranging from 27 to 47 years. Details of the patients were indicated in Table 4. Patient MY630304 was a male diagnosed with liver cirrhosis, secondary to chronic positive hepatitis B e Antigen (HBeAg +ve). The patient was initially treated with lamivudine before switching to tenofovir due to viral suppression failure. The HBV whole genome analysis of this patient also showed the presence of lamivudine resistant mutation in the P gene reported in the authors` previous publication (9). Patient MY853571 was a female diagnosed with chronic hepatitis B. The patient was treated with lamivudine for a period of two years (2009-2011). Patient MY62981 was also a female diagnosed with chronic hepatitis B. The patient had a family history of hepatitis B and HCC among two of the four siblings as well as the husband. Patient MY851636 was a female referred to the hospital for acute hepatitis B flare. The patient had presented jaundice, fatty liver, and intrahepatic cholestasis in pregnancy. Patient’s brother had passed away because of ascites when 38 years old. Patient MY131767 had chronic HBeAg negative, diagnosed since 2003 during blood donation. This patient did not have hepatitis B family history.
| Patient ID | Sex | Age | History oF Treatment | HBV Viral Load | HBV Genotype | HbsAg; Status | Clinical Diagnosis |
|---|---|---|---|---|---|---|---|
| MY630304 | M | 46 | -Initially on Lamivudine since June; 2009, later switched to tenofovir from July 2011 till May 2012 due to failure of viral suppression | -Before treatment: 7.6 × 107 IU/mL; -Last reading before; switching to tenofovir:1.59 × 107 IU/mL; -Three months; after receiving tenofovir: 1.0 × 103 IU/mL; -One year after; receiving tenofovir: < 6 IU/ml | C | positive | -Liver cirrhosis secondary to Chronic Hepatitis B; -HBeAg+ve; -Pt keeping; well and no signs of liver decompensation after receiving; tenofovir |
| MY62981 | F | 47 | -On lamivudine from 2009-2011 | In April 2011: < 6.0 IU/mL In April 2012: 7663IU/mL In June2013: 5896IU/mL | D | Positive | -Chronic Hepatitis B; -HBeAg negative; -No cirrhosis; -SG Abdomen showed liver diffusely increased in echogenecity, no focal lesion, normal in size, no ascites; - Fatty liver |
| MY853571 | F | 27 | No treatment recorded | In September 2013: 9.9 × 107IU/mL | B | positive | Chronic Hepatitis B; HBeAg +ve; Has family history of HCC and Hep B; Husband too has Hep B; US Abdomen showed normal hepatobiliary system and tiny non obstructive calculus in left lower pole |
| MY851636 | F | 31 | No treatment recorded | NA | C | positive | -Referred for acute Hep B flare; -No liver decompensation; Fatty liver in pregnancy; Intrahepatic Cholestatic in pregnancy; Jaundice; Family history: Brother had ascites and passed away |
| MY131767 | F | 34 | No treatment recorded | Nov 2010: 14291 IU/mL July 2012: 32460 IU/mL March 2013: 44172IU/mL | B | positive | -Chronic Hepatitis B; HBeAg negative; No family history of Hep B/ HCC; -No liver cirrhosis |
Characteristics of Patients With Deletion at ORF of Pre-S1 a
4.5. BCP, PC and C Region Analysis
Analysis of all sequences with complete C gene region showed the presence of several important mutations (Table 5). The most common mutation observed was precore mutation C1858 T which comprised 64.5% of the sequencedisolates. Another identified precore mutation was G1896A with the occurrence of 25.8%. Among the basal core promoter mutations, A1762T-G1764A double mutation was present in 26.9%, C1653T in 8.6%, A1752G in 10.8% and C1766T in 2.2% of the isolates.
| C Gene Mutations | Prevalence of Occurrence |
|---|---|
| C1653 T | 8.6 |
| A1752 G | 10.8 |
| 1762 AGG-TGA 1764 | 26.9 |
| C1766 T | 2.2 |
| T1768 A | 10.8 |
| C1858 T | 64.5 |
| G1896 A | 25.8 |
5. Discussion
In the current study two significant mutations were identified in the S gene of HBV isolated from Malaysian hepatitis B carriers. The most common mutation in the S region was the pre-S1 deletion which leads to an inefficient immune response (10, 11). The occurrence of pre-S1 deletion in the current study population was significantly more prevalent in the female patients (4.5; 80%). This result was different from that of another study which demonstrated that pre S deletions (pre-S1 and pre-S2) were detected in a higher frequency in male (12). No clear association with genotype was found in this study. However, evidence that preS1 deletion is inherent in genotype D may support the current study findings whereby one of the five patients with pre S1 deletion was infected with HBV genotype D (13, 14). In addition, the length of the pre S1 deletion (33 nt) in the genotype D patient was longer than those of the other genotypes. This finding was similar to several studies which reported that genotype D of HBV has the characteristic of 33 nt deletion (15-17). The present study demonstrated that pre-S1 deletion mutants of HBV occurred in 80% (4.5) of the individuals with chronic hepatitis B infection. Only one was diagnosed with acute Hepatitis B. There was a similar finding that underscored the emergence of pre-S mutations in the chronic infections (17). The pre-S regions play an essential role in the interaction with the immune responses because they contain several epitopes for T or B cells, therefore deletion in this region seems to represent desperate escape of HBV from the host immune surveillance. Many studies have reported the association of pre-S1 deletion with HCC and progressive liver disease (12, 18, 19). There was no such association in the current study. In contrast, a study showed that pre-S1 deletion was common in asymptomatic HBV carriers (ASC) (14). A reported meta-analysis study indicated that the frequency of pre-S1 deletion consecutively increased during progression of chronic hepatitis B from ASC states to liver cirrhosis (LC) or HCC (13). Therefore asymptomatic chronic HBV carriers with early detection of pre-S1 deletion should not be neglected and need to be included in the surveillance programs for HCC. of the five patients with HBV pre-S1 deletion had a family history of hepatitis B suggesting that the variation might be inherited. However there was little evidence supporting that pre-S1 deletion was transmissible. The occurrence of another mutation W182* was identified in the present study in two HBV patients. This mutation is known to cause a premature termination in the pre-S1 region which subsequently prevents the expression of S gene full length. Association of HBV genotypes and occurrence of W182* mutant is not clearly known. The current study findings revealed that both patients with this mutation were infected with HBV genotype C suggesting the probable association of genotype C and presence of W182* mutant. One of the patients showed progression of liver disease to HCC complicated with bleeding within a few months. The other patient could not be followed up, thus the current status is unknown. This crucial finding suggests that W182* could serve as a molecular marker to predict HCC. Other studies have also reported that the presence of W182* mutation in patients with Hepatitis Bcaused progressive forms of the disease (HCC and liver disease) (20, 21).Hepatitis B virus can evolve by mutations in the major hydrophilic region (MHR) of the HBsAg which allows its escape from host immune responses (22). The MHR region contains residues 99-169 of HBsAg and T/I126S, Q129H, G130N, S143L, D144A, G145A, and G145R were among the commonly reported mutations. However none of these mutations were detected in the current study. In conclusion, genomic analysis of the of HBV S gene isolated from Malaysian HBV carriers revealed the occurrence of two important variants,pre-S1 deletion and W182* mutant. The pre-S1 deletion was highly prevalent in female with chronic hepatitis B and with family history of hepatitis B. The W182* mutant was present in HBV genotype C and patients who showed progression to HCC. These findings will add more information to the current database of the clinically significant mutations in Malaysian Hepatitis B isolates. It would be interesting to conduct continuous surveillance among Malaysian hepatitis B carriers for the presence of these mutations along with other S gene mutations to establish probable association with genotypes, clinical symptoms or progression of hepatitis infection.