1. Insulin Resistance as a Bridge Between Hepatitis C Virus and Diabetes
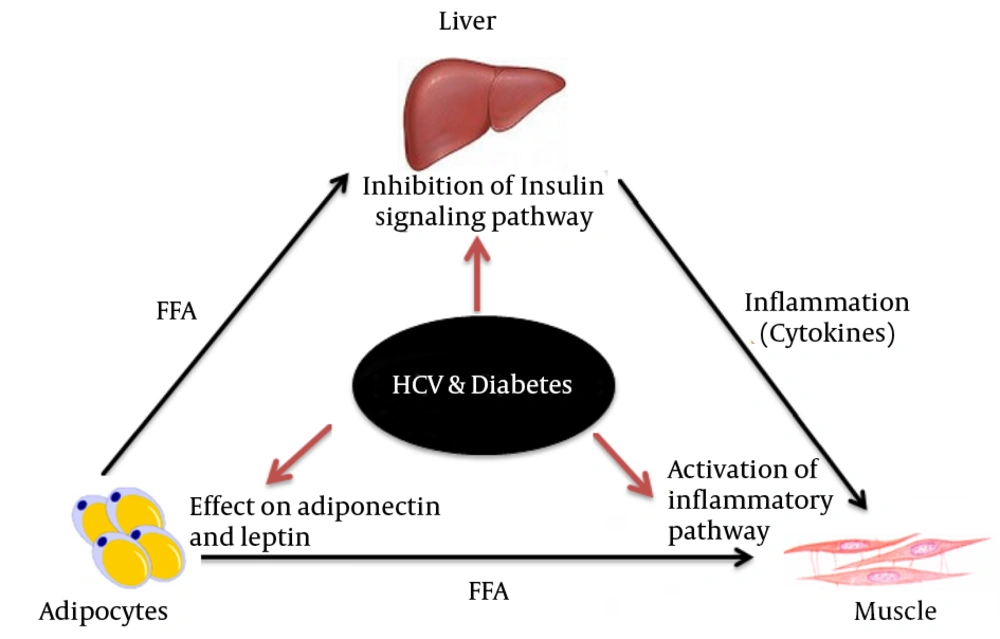
Both diabetes and hepatitis C virus (HCV) infection are severe health problems worldwide, especially in the developing countries (1, 2). A range of extrahepatic (EH) manifestations such as arthralgias, thyroiditis and diabetes are linked with HCV infections (3-5). Studies have shown that patients infected with hepatitis C virus (HCV) have more glucose intolerance than the general population. According to a report, up to one-third of patients with chronic liver disease caused by hepatitis C virus (HCV) infection develop type II diabetes mellitus (6). It is well documented that the levels of reactive oxygen species (ROS) are higher in both diabetic and HCV infected patients (7, 8). This potential synergism of HCV and diabetes is attributed to the multifaceted interactions between HCV and glucose metabolism. In our previous study, we showed that HCV may also cause hepatic steatosis, even though the clinical impact of viral steatosis is still debated (9). Among all factors, insulin resistance seems to be a vital feature of the pathogenesis of HCV-induced glucose intolerance. Several mechanisms may account for the development of insulin resistance in patients with chronic HCV infection. For instance there is evidence for a triangular interaction between insulin resistance, steatosis and inflammatory cytokines (Figure 1). This triangle of interactions means that insulin resistance associated with diabetes or HCV, can promote fatty liver (steatosis) and fat accumulation may in turn promote insulin resistance and inflammation. HCV promotes dysfunction of insulin signaling pathways via several distinct mechanisms. One of our recent studies on HIT-T15 cells, cultured under hyperglycemic conditions demonstrated increased insulin resistance with a significant increase in the levels of MAPK, NF-κB and IRS-1 serine phosphorylation (ser307) and decreased Akt and insulin contents (10). Similarly, studies demonstrated that HCV infection also induces insulin resistance through impairment of IRS-1 and AKT, in particular, increasing the IRS-1 phosphorylation at serine residues and decreasing it at tyrosine residues (11, 12). Furthermore, HCV up-regulates the expression of suppressors of cytokine signaling 3 (SOCS3) and tumor necrosis factor-α (TNF-α), while down-regulates peroxisome proliferator-activated receptors gamma (PPARγ) (13, 14). HCV genotype 1 usually affects IRS-1 through SOCS-3 mediated ubiquitinylation, while HCV genotype 3 diminishes IRS-1 via enhanced expression of SOCS-7 (14, 15).
The association between HCV and diabetes was initially perceived as a non-specific consequence of hepatic inflammation. To verify this view, Shintani et al. (16) conducted a study by expressing HCV core protein in transgenic mice. HCV core protein resulted hepatic insulin resistance in the transgenic mice; however, HCV core protein-induced insulin resistance was reversed by administration of antibodies against TNF-α. Due to enhanced levels of interleukin (IL)-1, interleukin (IL)-6, Leptin and tumor necrosis factor (TNF)-α and reduced levels of adiponectin, chronic inflammation plays a substantial role in the development of insulin resistance. These inflammatory cytokines stimulate the inflammatory mediator IκB kinase β (IKKβ) and IKKβ induces the proteasomal degradation of anti-inflammatory inhibitor of NFκB (IκB) at serine 32 and 36, allowing nuclear translocation of the downstream effector molecule NFκB to promote insulin resistance (17-19). The same happened in our study (unpublished) where hyperglycemia increased phosphorylation of IκB at serine 32 and 36 in Human umbilical vein endothelial cells (HUVECs).
2. Risk Factors
Studies indicated that family history of diabetes, obesity and severe liver fibrosis are the key risk factors for developing both HCV and diabetes concomitantly. Atherosclerosis patents have been reported at greater risk for developing HCV and diabetes. Literature also showed that interferon therapy might have some implications in the development of diabetes in HCV infected patients; however, this association is quite rare and cannot be regarded as potential risk factors. Furthermore, insulin sensitivity in non-diabetic HCV patients has revealed significant association with histological activity index, serum aspartate aminotransferase and the degree of fibrosis (20).