1. Background
Hepatitis C virus (HCV) is an enveloped, single-stranded ribonucleic acid (RNA) virus with positive polarity. This virus is one of the most important pathogens of the human and is able to cause mild to severe liver diseases. The virus is a member of the hepaciviruses genus in the Flaviviridae family (1). Hepatitis C virus infection is a major blood-borne infection worldwide, with a silent epidemiology, that it has reached pandemic proportions (2). The chronic infection with HCV, remains a troublesome health problem worldwide, which approximately 3% of the population suffering from it. Its prevalence is higher in some countries of Asia and Africa and approximately 14.5% in Egypt (3-5). In developing countries, the chronic hepatitis C is the most prominent cause of liver cirrhosis, hepatocellular carcinoma and liver transplantation (6). However, the epidemiological pattern of HCV is still obscure (7, 8). Available estimates indicate that worldwide there were 54,000 deaths and 955,000 disability- adjusted lifeyears associated with acute HCV infection (4). The main routes of transmission in HCV are exposure to infected blood or blood product, intravenous drug use, infected medical equipment, tattooing, needle stick, hemodialysis, sexual activity and organ transplantation (2, 3, 5).
The prevalence of anti-HCV from population-based studies is used to compare HCV infection levels globally. Hepatitis C virus infected an estimated 185 million people worldwide and is a significant cause of morbidity and mortality. Also, in developed countries, HCV predominantly infects people who inject drugs (9). Peoples in some closed settings such as prisons, jails, juvenile detention facilities, pretrial detention centers, and extrajudicial detention centers for people who use drug showed higher prevalence of HCV (10). However, it seems that the prevalence of this virus in the general population of Iran is less than 1% (11), which is approximately lower than the reports of the neighbor countries. The prevalence of HCV in the general population of different countries of the developed world around Iran has been reported to be between 0.9 and 4% (11). The difference observed in different populations may be due to laboratory and selection methods. Also, it seems that there are differences between urban and rural population. There are no previous reports about the prevalence of HCV in rural population of Iran and due to the differences in HCV-related risk factors between these two populations, performing of the study on the prevalence of HCV in the rural population is seriously needed.
2. Objectives
This study was designed to determine the prevalence of anti-HCV antibody in general population of two villages, Farmashkan and Akbarabad, of the Kavar city in Fars Province, Iran, and evaluate the related risk factors in these areas. Moreover, the possible associations between all risk factors with anti-HCV antibody prevalence were also evaluated in this population.
3. Patients and Methods
The present study is a part of Kavar cohort study (K.C.S), which is started from 2006 in Kavar town with about 71856 populations. This town is located in 35 kilometer southeast of Shiraz, the capital of Fars Province, Iran. Gastroenterohepatology and Endocrine Research Centers affiliated to Shiraz University of Medical Sciences are implementing K.C.S. From the start of K.C.S till now, all peoples are followed every two years. In the original cohort study, demographic and anthropometric characteristics of participants are documented in the questionnaire. In a cross-sectional study, which approved in Ethical Committee of Shiraz University of Medical Sciences, by using Cochran formula and assuming p = 0.03, q = 0.97, d = 0.01 and α = 0.95, the sample size of 1049 was calculated. Statistical power for this study was 0.95. For better performing of the study we used census method sampling and therefore, blood samples of all of the Iranian peoples aged ≥ 7 years old in the two villages, Farmashkan and Akbarabad of the Kavar City in Fars Province, Iran, which referred to the K.C.S Center were used. Our inclusion criteria were age equal or more than 7 years and using no medication for the HCV treatment. Our study had no exclusion criteria except voluntary withdrawal from the study. Demographic information included age, gender, marital status, occupation, history of blood and its product transfusion, hemodialysis, organ transplantation, dental procedure, accident, war injury, tattooing, injection drug use, addiction, alcohol use, personal or family history of liver disease, imprisonment, extramarital sexual contact, hepatitis B virus (HBV) vaccination, concomitant diseases and its symptoms were extracted from their medical records.
The blood samples (10 mL) were cooled on ice and taken to a specialized laboratory affiliated to Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. The blood samples were centrifuged at 3500 rpm for 15 min at 4°C and the serum was separated and stored at -70°C until further analysis. The serum IgG antibody against HCV was determined using a third-generation indirect enzyme immunoassay (EIA) kit (Acon Laboratories, Inc, USA). This test can detect various subtypes of HCV antibodies using the recombinant antigens specific for core, NS3, NS4 and NS5. The clinical sensitivity and specificity of this kit are > 99.9 % and 99.8 %, respectively.
3.1. Statistical Analysis
Data were reported as frequency and percentage and analyzed using SPSS software version 17.0 (Washington, USA) for Windows. Qualitative data were analyzed by chi-square and Fisher’s exact tests to find any association between risk factors and HCV-antibody positivity. An independent samples t test was used to compare the age variable between the two villages. A P value < 0.05 was used to indicate a statistically significant difference.
4. Results
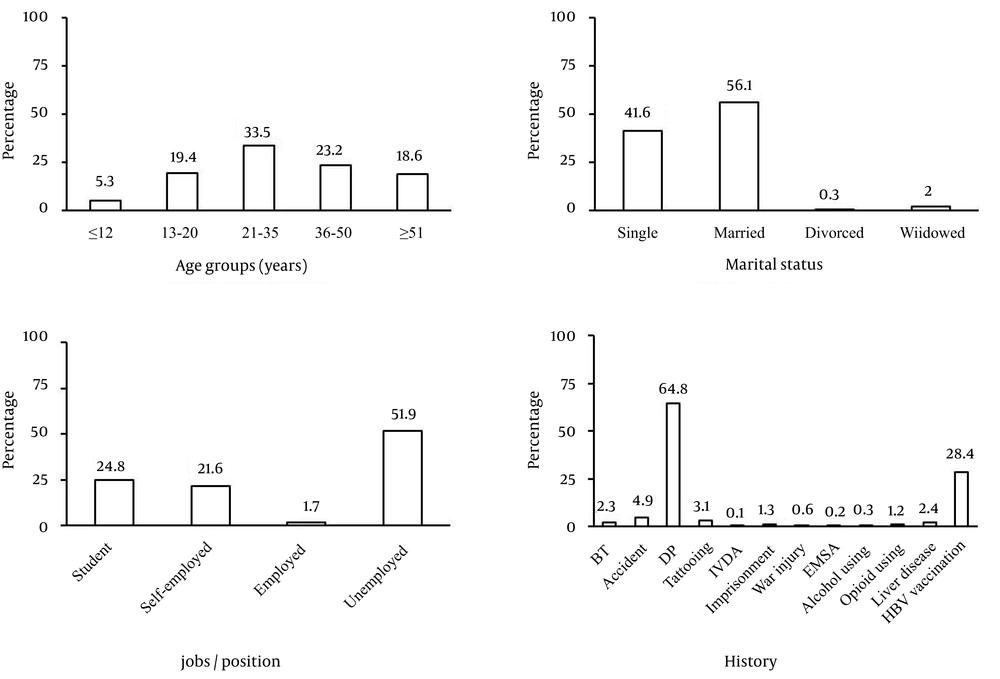
A total of 6095 participants with age range of 88 years (7-95 years) and mean ± standard deviation of 34.58 ± 17.29 years, including 2218 males (36.4%) and 3877 females (63.6%) were enrolled in this study. A statistically significant difference was observed regarding the age of persons between the two villages (3393 and 2702 participants with age of 32.62 ± 16.52 and 36.93 ± 17.90 years from Akbarabad and Farmashkan, respectively, P < 0.001). Age groups, marital status, jobs/positions and other historical information are presented in Figure 1. Most of our participants were in the age range of 21-35 years (33.5%), married (56.1%), and unemployed (51.9%) and had the history of dental procedure. Overall, 15 out of the 6095 participants, 8 female and 7 male subjects, were anti-HCV positive (0.25%). Although, anti-HCV antibody prevalence was two in 1000 participants; however, seroprevalence of HCV in children was four times greater than general population and was 1%. Frequency and percentage of the person’s anti-HCV antibody status based on the age groups, marital status, jobs/position and related risk factors are presented in Table 1. Among them, 12 persons were symptom-free and only 3 persons were afflicted with fatigue and mild anorexia. One of them also had abdominal pain. Despite the HBV vaccination, two children (13.3%) had the concomitant Hbs-Ag positivity and HBc-Ab negativity.
| Frequency of Anti-HCV Antibody | P Value | ||
|---|---|---|---|
| Positive | Negative | ||
| Villages | 0.467 | ||
| Akbarabad | 12 (0.3) | 3393 (99.7) | |
| Farmashkan | 3 (0.1) | 2702 (99.9) | |
| Gender | 0.428 | ||
| Male | 7 (0.3) | 2211 (99.7) | |
| Female | 8 (0.2) | 3869 (99.8) | |
| Marital status | 1.000 | ||
| Single | 6 (0.2) | 2530 (99.8) | |
| Married | 9 (0.3) | 3412 (99.7) | |
| Divorced | 0 (0) | 19 (100) | |
| Widowed | 0 (0) | 119 (100) | |
| Age, y | ND | ||
| ≤ 12 | 3 (1.0) | 310 (99.0) | |
| 13-20 | 3 (0.3) | 1150 (99.7) | |
| 21-35 | 2 (0.1) | 1985 (99.9) | |
| 36-50 | 4 (0.3) | 1369 (99.7) | |
| ≥ 51 | 3 (0.3) | 1099 (99.7) | |
| Jobs/positions | ND | ||
| Student | 5 (0.3) | 1484 (99.7) | |
| Self-employed | 4 (0.3) | 1293 (99.7) | |
| Employed | 0 (0) | 101 (100) | |
| Unemployed | 6 (0.2) | 3101 (99.8) | |
| Blood transfusion | 4 (2.8) | 139 (97.2) | < 0.001 |
| Accident | 1 (0.3) | 296 (99.7) | 0.528 |
| Dental procedure | 12 (0.3) | 3940 (99.7) | 0.285 |
| Tattooing | 0 (0) | 189 (100) | NP |
| Intravenous drug abuse | 0 (0) | 4 (100) | NP |
| Imprisonment | 1 (1.2) | 81 (98.8) | 0.184 |
| War injury | 0 (0) | 37 (100) | NP |
| Extramarital sexual activity | 0 (0) | 15 (100) | NP |
| Alcohol using | 0 (0) | 18 (100) | NP |
| Opioid using | 0 (0) | 75 (100) | NP |
| Liver disease | 0 (0) | 149 (100) | NP |
| Dialysis | 0 (0) | 0 (0) | NP |
| Organ transplant | 0 (0) | 0 (0) | NP |
a Abbreviations: ND; not determined, NP; not performed.
bData are presented as No. (%).
Comparisons of the effects of gender, marital status, age and history of blood transfusion, dental procedure, accident and imprisonment on the frequency of anti-HCV antibody are presented in Table 2. Only a significant relationship was detected between a history of blood transfusion and the prevalence of HCV (P < 0.001). Also, persons with history of blood transfusion had 15-fold higher risk for anti-HCV positivity (OR: 15.54, 95% CI: 4.89-49.41).
| Frequency of Anti-HCV antibody | P value | ||
|---|---|---|---|
| Positive | Negative | ||
| Gender | 0.428 | ||
| Male | 7 (0.3) | 2211 (99.7) | |
| Female | 8 (0.2) | 3869 (99.8) | |
| Age, y | 0.226 | ||
| ≤ 20 | 6 (0.4) | 1460 (99.6) | |
| > 20 | 9 (0.2) | 4453 (99.8) | |
| Marital status | 1.000 | ||
| Single | 6 (0.2) | 2530 (99.8) | |
| Married | 9 (0.3) | 3550 (99.7) | |
| Blood transfusion | < 0.001 | ||
| Yes | 4 (2.8) | 139 (97.2) | |
| No | 11 (0.2) | 5941 (99.8) | |
| Dental procedure | 0.285 | ||
| Yes | 12 (0.3) | 3940 (99.7) | |
| No | 3 (0.1) | 2140 (99.9) | |
| Accident | 0.528 | ||
| Yes | 1 (0.3) | 296 (99.7) | |
| No | 14 (0.2) | 5784 (99.8) | |
| Imprisonment | 0.184 | ||
| Yes | 1 (1.2) | 81 (98.8) | |
| No | 14 (0.2) | 5999 (99.8) | |
a P value < 0.05 is considered as significant.
b Data are represented as No. (%).
Among two villages, 3393 and 2702 out of the 6095 participant were native of Akbarabad and Farmashkan with the age range of 83 and 88 years (7-90 and 7-95 years) and the mean age of 32.62 ± 16.52 and 36.93 ± 17.90 years, respectively. Moreover, the prevalence of persons with positive anti-HCV antibody was 0.35 and 0.11%, respectively. A statistical significant association was found between blood transfusion and anti-HCV antibody positivity in Akbarabad village (P < 0.001). Our analysis also demonstrated that in the Akbarabad village, those with the age ≤ 12 years old had three-fold higher risk for anti-HCV antibody positivity than other subjects (OR: 3.15, 95% CI: 0.95-10.44). In addition, the individuals with a history of blood transfusion had 46-fold higher risk for anti-HCV antibody positivity than those with negative history of blood transfusion (OR: 46.41, 95% CI: 11.18-192.72).
No statistical association was found between the anti-HCV antibody positivity and evaluated variables except history of blood transfusion (P < 0.001) in Farmashkan villages (P > 0.05). Individuals with a history of blood transfusion had11-fold higher risk for anti-HCV antibody positivity than those with negative history of blood transfusion (OR: 11.40, 95% CI: 1.02-127.21).
5. Discussion
This study calculated the prevalence of HCV infection in the general population of the two Kavar villages, Akbarabad and Farmashkan, and assessed its association with gender, age, marital status, jobs/position and history of some related risk factors. Our participants were rural general populations and most of them were without any known HCV-related risk factors. The general prevalence of HCV in this study was detected as 0.25% and the highest prevalence was seen in age ≤ 12 years old (1%). Although there was a significant association between blood transfusion and anti-HCV antibody positivity, no significant associations were detected between other related risk factors and anti-HCV positivity.
Based on a systematic review in 2009, HCV infection prevalence in general population was calculated as 1.6% (3) that is much higher that our results. In another study, which performed in the civilian population of the United States, it had been demonstrated that the prevalence of anti-HCV in the United Sates decreased from 1.9% in 2001to 1.3% in 2005, and remained stable up to 2010 (12). The total viremic HCV population of Australia in 2012 was estimated at 230000 with a viremic prevalence rate of 1.0%. In addition, the anti-HCV prevalence was estimated at 1.3%, equivalent to 308000 anti-HCV positive individuals (13). Ferthermore, several studies were performed about the prevalence of HCV in the European, American, African and Asian countries. In a study from Austria in 2008, the anti-HCV prevalence rate of 0.5% (0.1–0.7%) was reported (14). In Belgium, different low (0.12%) and high (1.23%) prevalence rates were reported in two studies performed in 2007 (15) and 2012 (13), respectively. Total seroprevalence of 1.38, 0.96, 0.6,0.63, 12.5, 0.4, 0.5, 2.6, 1.6 and 0.95 % in Brazil (16), Canada (17), Czech Republic (18), Denmark (19), Egypt (20), England (21), Germany (22),Spain (23), Switzerland (24) and Turkey (25), were also recorded, respectively. Our detected HCV prevalence is lower than all previous reports and approximately near to the reports from England and Germany. Also, our prevalence is similar to a recently report from Mashhad, Iran. In that study, the prevalence of hepatitis C seropositivity in the general population of Mashhad, northeast of Iran, was evaluated and its results demonstrated that the overall seroprevalence of hepatitis C was 0.2% using the ELISA method (1). Merat et al. reported that the HCV prevalence in Iran is 0.5% in individuals with age range of 18-65 years and higher and about 1% was seen in male (26). This prevalence rate is two-fold higher than our results. The probable reason for this difference may be the nature of two population, the urban population in Merat et al. (26) and Shakeri et al. (1) studies and the rural population in our study. Therefore, the HCV infection related risk factors and consequently anti-HCV antibody prevalence were lower in this study. However, the HCV viral load evaluation using RT-PCR is highly recommended to confirm this difference.
Although, the prevalence of HCV infection in children ranges from 0.05 to 0.4% (27), the higher prevalence was seen in this study for the persons with age of ≤ 12 years old (1%). It is necessary to evaluate the HCV antibody of both children and their mothers to rule out the vertical transmission of HCV. In our study, similar to previous reports from Iran (1, 26), the HCV-positive male was dominant in comparison to female (0.3% vs. 0.2%, respectively). However, this difference was not significant and therefore there was no association between gender and anti-HCV antibody positivity.
In an Iranian study performed by Kohan et al. in 2006, 11.1% of HCV infected persons (23 out of 207) co-infected with HBV (28). In our study, the HBV-HCV co-infection was detected in 13.3% of the persons (two out of 15). These two children were HbsAg positive and HBcAb negative. Also, the high anti-HCV antibody positivity was detected in the student groups (24.8%) based on the job/position categorization. This can be a warning for Education and Health Ministry for learning mostly about viral hepatitis transmission routes and prevention, especially HBV and HCV.
Transfusion of the infected blood and blood product is one of the major routes of the HCV infection. This is more important in some populations such as thalassemia, hemophilia, and hemodialysis because they are dependent on the blood products during their lifetime. It has been demonstrated that the HCV infection rate in these groups is higher than the normal population (29-40) as demonstrated in our study.
Our study suffered from some limitations including limitations in the univariate analysis and some limitations related to the effects of a long-time of the cross-sectional study. Also, lack of confirmation of the positive anti-HCV antibody samples with molecular methods, such as PCR and RT-PCR is another limitation. However, this issue is being set up and performed in our center for larger cohort study.
In conclusion, due to this fact that HCV infection is a preventable and curable disease, we recommend more attention for the control of patient-to-patient HCV transmission in hospitals. Although prevalence rate in this study was lower than other studies and no statistical significant associations were found with common risk factors except blood transfusion, paying the especial attention to the all risk factors is seriously advised. Finally, further studies are required in other rural and urban populations for better evaluation of the HCV-infection prevalence and real source of transmission.