1. Background
Hepatitis has been described as a medical illness defined by the inflammation of the liver and manifested by the appearance of inflammatory cells in the liver tissues (1, 2). Generally speaking, the clinical symptoms of hepatitis include poor appetite, nausea or vomiting, diarrhea, jaundice and malaise (3, 4). It has been estimated that chronic hepatitis B virus (HBV) has affected approximately 360 million people, and 5.7 million cases with diseases related to HBV have been diagnosed around the world (2). In addition, the projected incidence of hepatitis C virus (HCV) is illustrated to be 2.2% worldwide, with 3 to 4 million new cases, each year (2, 5), and there are also about 15 million people infected with hepatitis D virus (HDV), globally (6). Despite hepatitis viruses, which have been considered as the well-established cause of hepatitis, other relevant factors may also conduce to the pathogenesis of hepatitis (7, 8). These factors may include infections, autoimmune diseases and toxic substances, such as alcohol, several medications, certain industrial organic solvents and plants, etc. (7, 9, 10). Recently, cytokeratin-18 (CK-18), which is important for hepatocyte integrity, has been demonstrated to be implicated in the development of hepatitis (11, 12).
The CK-18, a main intermediate filament protein found in liver cells, belongs to the intermediate filament family consisting of approximately 70 cytoskeleton molecules (12). The CK-18 participates in cellular processes, including mitosis, responses to stresses and cell cycle progression (12-14). It is widely accepted that CK-18 is laminated by caspases at two conserved residues, during the period of apoptosis, one of which (Asp 396) is a neoepitope (M30), which is not observed in vital or necrotic cells, although it is strengthened in a variety of liver illness (15). The full-length variant of CK-18 is regarded to arise from necrotic cells, while the caspase-laminated form of this protein is released from cells undergoing the process of apoptotic death (16). There is evidence revealing that serum levels of apoptotic CK-18 neoepitope increase in patients infected with HCV (17). Consequently, apoptotic CK-18 has been supposed to be an important marker in the early evaluation of liver disruption, before the disruption is morphologically evident in hepatocytes (18). Furthermore, serum CK-18 levels have also been utilized to quantify apoptosis in liver disorders, like non-alcoholic steatohepatitis (NASH) and HCV infection (19, 20). It is a well-known fact that hepatocyte apoptosis plays an essential role in the pathogenesis of HCV, which may be also closely linked to liver fibrogenesis (21, 22). More importantly, it has been indicated that disruption of liver cells in HCV infection is modulated by induction of apoptosis, which was considered as morphopathologic feature of infected hepatocytes in individuals (15, 18, 21). Actually, apoptosis of infected cells may represent a cellular defense mechanism in the prevention of viral propagation. However, several viruses may try to damage the normal modulation of infected cell death so as to avoid this defense and decrease the response to apoptosis (15). From all the aspects, we postulated that serum CK-18 levels may be implicated in the progress of hepatitis. Several studies held the view that serum CK-18 levels might be a useful biomarker in assessing liver damage in hepatitis (23, 24), while other documents failed to support this finding (11, 25).
2. Objectives
The objective of the present meta-analysis was to clarify the connection between serum CK-18 levels and hepatitis pathogenesis.
3. Materials and Methods
3.1. Literature Search
With the application of computerized databases [PubMed, Embase, Cochrane Library, Google Scholar, Web of Science, China BioMedicine (CBM), China National Knowledge Infrastructure (CNKI)], published papers, which assessed the association between serum levels of CK-18 and hepatitis susceptibility, were obtained. “Hepatitis”, “hepatitides”, “hepatitis D, chronic”, “Keratin-18”, “KRT18 protein, human”, “Keratin 18” , “Endo-B Cytokeratin”, “CK-18” were entered as common keywords, with a highly sensitive search strategy. No restriction of language was set. We also further looked through the bibliographies manually, to identify additional potential relevant papers.
3.2. Study Selection
Published papers eligible for this meta-analysis had to meet the following inclusion criteria: (1) case-control studies based on a human population to examine the association between serum levels of CK-18 and hepatitis susceptibility; (2) patients pathologically confirmed with hepatitis; (3) articles published with full text; (4) studies with sufficient information on the serum CK-18 levels in the cases and the controls. The major exclusion criteria were as follows: (1) did not satisfy the inclusion criteria; (2) letters, case report, reviews, proceedings or meta-analyses; (3) repeated publications or studies with overlapping data.
3.3. Data Extraction and Quality Assessment
We used a standard reporting form to extract data from each included study, and the following relevant data were extracted from eligible studies, prospectively, in the final analysis, even though several articles did not contain all the data: surname and initials of the first author, year of publication, country, racial descent, number of cases and controls, age and sex of subjects, type of hepatitis, detection method of CK-18, CK-18 serum levels in the cases group and controls group. Two reviewers independently assessed the methodological quality of the included trials, based on the Newcastle-Ottawa Scale (NOS) criteria (26). Specifically, the NOS criteria included three aspects: (1) subject selection: 0-4; (2) comparability in subjects: 0-2; (3) clinical outcome: 0-3. Total NOS scores ranged from 0 to 9, with low quality (0-6) and high quality (7-9). Discrepancies on NOS scores of the enrolled articles were settled by discussion, or a third investigation was consulted.
3.4. Statistical Analysis
The association between serum levels of CK-18 and hepatitis susceptibility was estimated by the standardized mean difference (SMDs) with 95% confidence interval (CI). The Cochran’s Q-statistic (P < 0.05 was regarded as statistically significant) was used to evaluate the heterogeneity among studies. Fixed/random effects model was used to calculate the pooled SMDs. A P < 0.05 or I2 test exhibited > 50% indicated evidence of heterogeneity, and then the random-effect model was conducted, or else, the fixed-effects model was implemented (27, 28). Under the condition of heterogeneity existing, subgroup analyses of ethnicity, types of hepatitis were conducted to explore potential explanatory variables for the differences in serum levels of CK-18 between case group and control group. Even more, the one-way sensitivity analysis was carried out to evaluate whether one single study could have affected the overall estimation. Further, the funnel plot was used to assess possible publication bias, which might affect the accuracy of estimation. The symmetry of the funnel plot was evaluated using Egger’s linear regression test (P < 0.05 was considered significant). Statistical analysis was conducted by utilizing the STATA software, version 12.0 (Stata Corporation, College Station, TX, USA).
4. Results
4.1. Included Studies
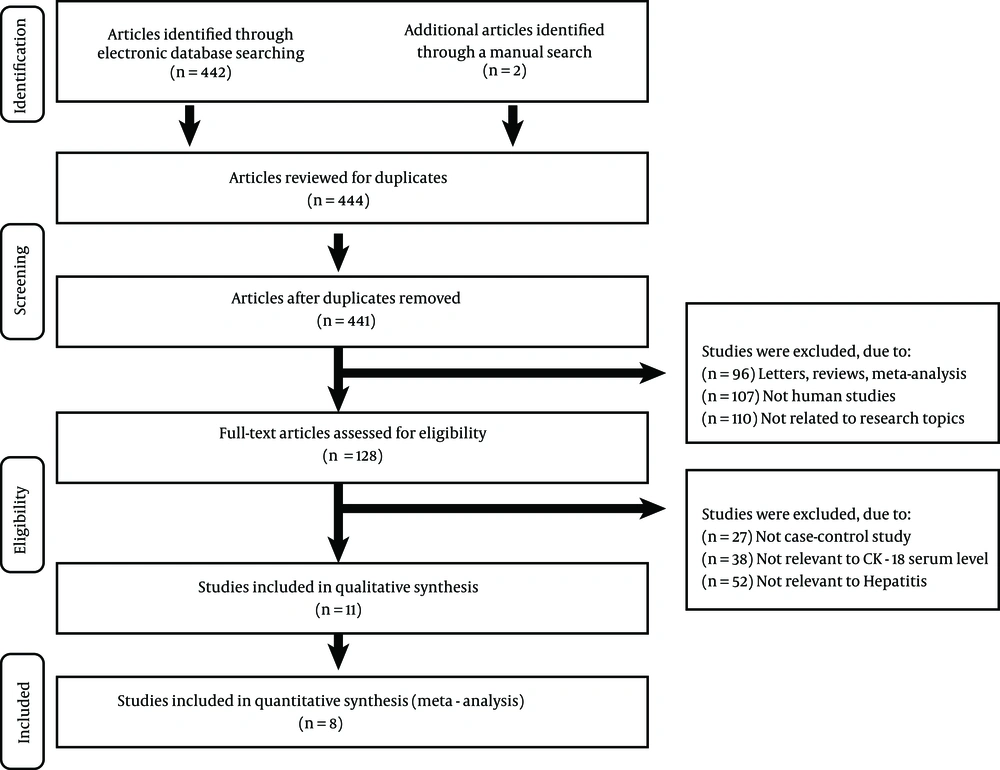
Figure 1 presented the steps of studies selection. A total of 444 reports were retrieved initially, through electronic database searching and manual search, and 128 papers were kept after removal of duplicates (n = 3), letters, reviews or meta-analyses (n = 96), non-human studies (n = 107), and the studies unrelated to the research topics (n = 110). Furthermore, 117 additional studies were excluded because they were not case-control or cohort studies (n = 27), not relevant to CK-18 serum levels (n = 38), or irrelevant to hepatitis (n = 52). In the final selection step, eight out of 11 studies were identified, with three articles being abandoned for not supplying enough information. These eight case-control studies, in full text, fulfilled our selection criteria, ultimately providing information on the correlation of serum CK-18 levels with hepatitis between 2010 and 2014 (8, 11, 15, 23-25, 29, 30). Demographic information on hepatitis patients, baseline characteristics and methodological quality of the enrolled studies are listed in Table 1. Three studies were performed in populations of Asians (China), four in Caucasians (Argentina, Greece, Spain, and Turkey), and one in Africans (Egypt), including 759 subjects, totally (515 patients with hepatitis and 244 healthy controls). As for disease type, three diseases, i.e. NASH, chronic hepatitis C (CHC) and chronic hepatitis B (CHB) were studied in our meta-analysis. Serum levels of CK-18 in hepatitis patients and controls were all detected with enzyme-linked immunosorbent assay (ELISA). All quality scores of the included studies were higher than 5 (high quality).
4.2. Serum Levels of CK-18 in Hepatitis
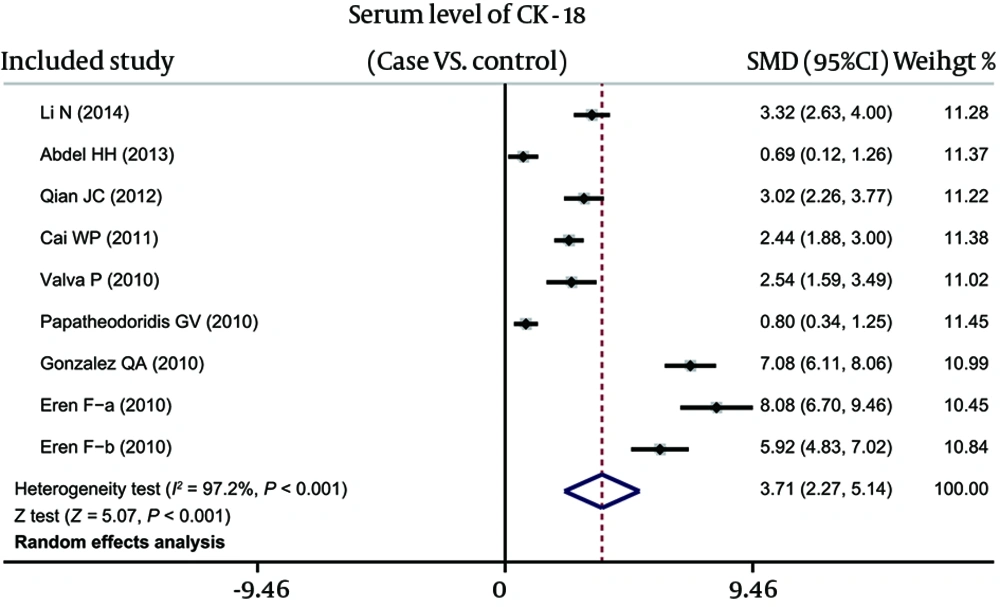
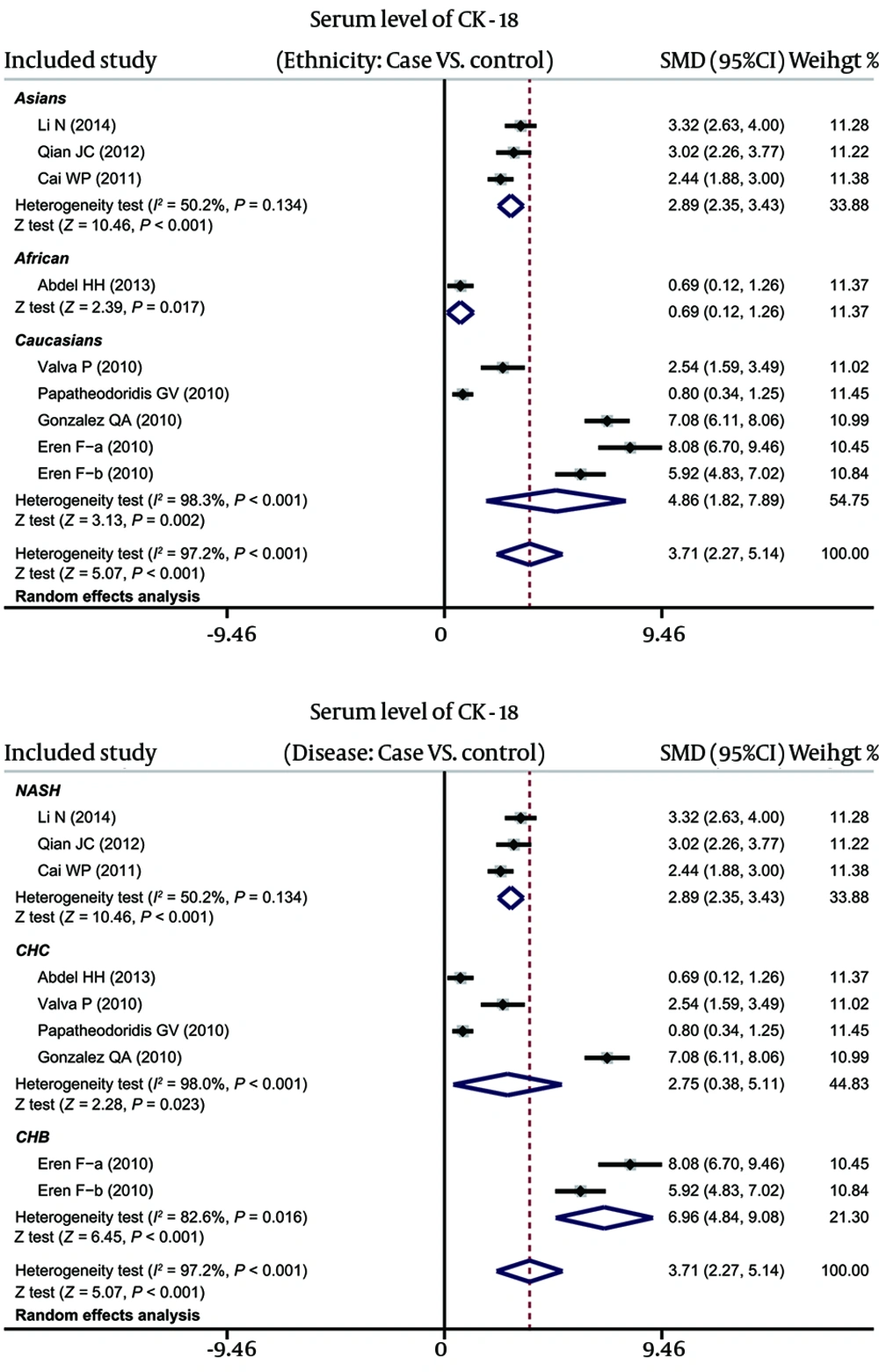
As shown in Figure 2, results revealed that the serum levels of CK-18 in hepatitis patients were higher than in healthy controls (SMD = 3.71, 95%CI: 2.27-5.14, P < 0.001). Subgroup analyses, based on ethnicity and disease, implicated that high serum CK-18 levels might be a risk factor for hepatitis in Asian (SMD = 2.89, 95%CI: 2.35-3.43, P < 0.001), African (SMD = 0.69, 95%CI: 0.12-1.26, P = 0.017) and Caucasian (SMD = 4.86, 95%CI: 1.82-7.89, P = 0.002), as well as NASH (SMD = 2.89, 95%CI: 2.35-3.43, P < 0.001), CHC (SMD = 2.75, 95%CI: 0.38-5.11, P = 0.023) and CHB subgroups (SMD = 6.96, 95%CI: 4.84-9.08, P < 0.001) (as shown in Figure 3).
4.3. Sensitivity Analysis and Publication Bias
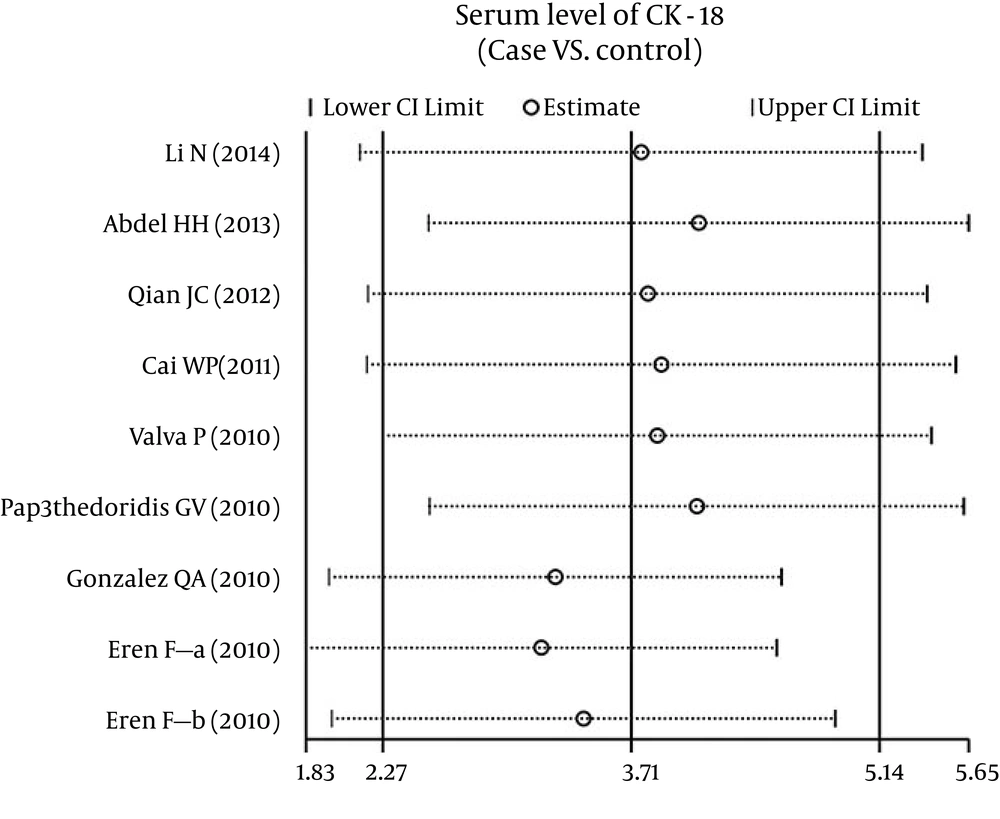
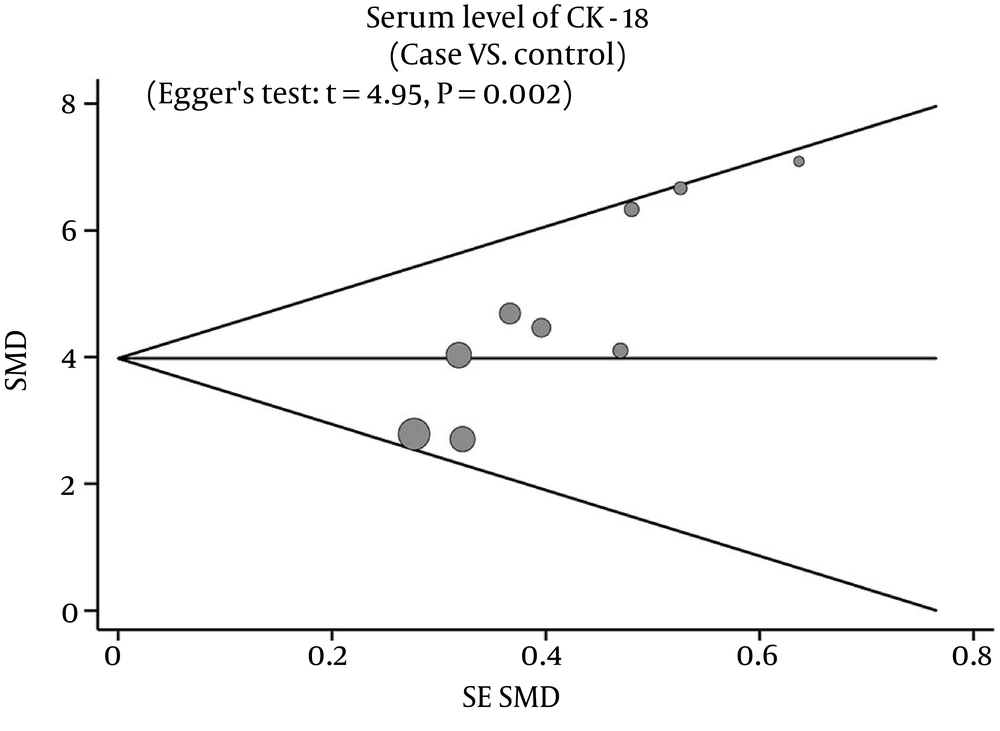
The overall statistical significance does not change when any single study was omitted. Therefore, the current meta-analysis data has stability and credibility (Figure 4). The graphical funnel plots of those eight studies presented relative asymmetry, while Egger’s test suggested the existence of publication bias (t = 4.95, P = 0.002) (Figure 5).
| First author | Year | Country | Ethnicity | Total | Sample Size | Gender, M/F | Age, y | Disease | Method | NOS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | ||||||||
| Li (23) | 2014 | China | Asians | 88 | 64 | 24 | 57/31 | 10.6 ± 2.4 | NASH | ELISA | 7 | ||
| Abdel Haleem (11) | 2013 | Egypt | African | 84 | 69 | 15 | 6/9 | 30.1 ± 9.4 | CHC | ELISA | 5 | ||
| Qian (24) | 2012 | China | Asians | 137 | 93 | 44 | 76/17 | 32/12 | 38.7 ± 10.9 | 37.9 ± 8.9 | NASH | ELISA | 8 |
| Cai (30) | 2011 | China | Asians | 87 | 47 | 40 | 31/16 | 26/14 | 45.6 ± 17.3 | 48.5 ± 18.7 | NASH | ELISA | 7 |
| Valva (15) | 2010 | Argentina | Caucasians | 34 | 23 | 11 | 8/15 | 1 ~ 17 | CHC | ELISA | 5 | ||
| Papatheodoridis (25) | 2010 | Greece | Caucasians | 92 | 62 | 30 | 45/17 | 13/17 | 48.0 ± 15.0 | 44.0 ± 14.0 | CHC | ELISA | 7 |
| Gonzalez-Quintela (29) | 2010 | Spain | Caucasians | 119 | 68 | 51 | 48/20 | 25/26 | 20 ~ 71 | CHC | ELISA | 7 | |
| Eren - a (8) | 2010 | Turkey | Caucasians | 76 | 47 | 29 | 21/26 | 17/12 | 45.9 ± 9.7 | 46.6 ± 9.3 | CHB | ELISA | 7 |
| Eren - b (8) | 2010 | Turkey | Caucasians | 42 | 42 | 22/20 | 17/12 | 26.4 ± 4.1 | 46.6 ± 9.3 | CHB | ELISA | ||
a Abbreviations: CHB, Chronic Hepatitis B; CHC, Chronic Hepatitis C; ELISA, Enzyme Linked Immunosorbent Assay; F, Female; M, Male; NASH, Non-Alcoholic Steatohepatitis; NOS, Newcastle-Ottawa Scale.
5. Discussion
Our meta-analysis was carried out with the aim to probe into the possible relationship between serum levels of CK-18 and the severity or stage of hepatitis. Through analysis from multiple aspects, we finally concluded that serum CK-18 concentrations are closely associated with hepatitis, and may even be used as a promising marker for detecting the possibility for hepatitis turning into hepatic fibrosis and hepatoma. Hepatitis is characterized by the symptoms of liver inflammation, induced by various viral infections, substance toxicity and autoimmune response, consequently leading to hepatocyte destruction, apoptosis or liver dysfunction (31). The CK-18, through its filament networks, is involved in several important cellular processes, including keeping stressors out of cells, maintaining cell cycle progression and controlling mitosis, particularly related to TNF-induced hepatocyte apoptosis, with cleaved CK-18 fragments by caspases (12). On the ground of these relative properties of hepatitis and CK-18, there were increasing reports speculating that CK-18 can be used as a noninvasive diagnostic marker for predicting the staging of chronic liver diseases. For example, a previous study has shown that CK-18 serum levels are increased in CHC development and the higher grades of chronic inflammation are correlated with overexpression of CK-18 within hepatocytes, suggesting that CK-18 has great predictive value in the diagnosis of hepatitis severity (11). Furthermore, the degree of liver damage because of hepatitis may decide fibrosis severity, which reveals elevated expression of CK-18 in CHB patients, and therefore CK-18 concentrations are implicated with the detection of liver fibrosis and has a significant utility in defining the pathological features of CHB patients, in the clinic (32). Additionally, there is a statement illustrating that the chief pathogenetic characteristics of hepatitis are related with the hepatocyte apoptosis process, originating from hepatocellular inflammation, necrosis and poisoning and induce the release of alanine aminotransferase (ALT); the role of CK-18 during apoptosis is dominantly manifested in the detection of ALT level and acts as an indicator of hepatocyte apoptosis, with usefulness for the diagnosis of hepatitis staging (8). On the account of the above explanations for the relationship of CK-18 levels and hepatitis, our meta-analysis results demonstrated that CK-18 serum levels are connected with the development and progression of various hepatitis diseases and may accurately evaluate their staging. Jazwinski et al. also put up with similar opinions, in agreement with our study results (33).
In addition, the subgroup analyses by ethnicity and disease were conducted, aiming to draw conclusions with superior accuracy and authenticity, via taking heterogeneities into consideration. The stratified analysis by ethnicity indicated that the research of CK-18 concentrations and hepatitis are strongly related among Asians, Africans and Caucasians. As for the effects of disease types, the evident results revealed that serum levels of CK-18 increased among CHB, CHC and NASH patients, all of which being involved in hepatic fibrosis aggravation, hepatocyte apoptosis and severe liver damage. Therefore, our study supported that serum CK-18 levels were up-regulated in hepatitis patients, which means the sensitive marker based on CK-18 levels has a significant value in detecting liver injury, diagnosing hepatitis grades and helpfully discriminating hepatitis from hepatoma.
Several specific limitations of this meta-analysis were advised to deserve careful consideration. Firstly, not all hepatitis participants underwent uniform diagnostic criteria, and especially the inclusion of three different types of hepatitis (CHB, CHC and NASH) lacked differential diagnostic criteria, and all of them would negatively effect on the reduction of possible misclassification. Secondly, a relatively small sample size of hepatitis patients were recruited in the screening setting, which might possibly influence the assessment of test sensitivity of detecting hepatitis. Thirdly, we only evaluated the relationship of CK-18 with the development of hepatitis, based on a single moment blood test. A multiple time blood test or stool-based tests with more apoptosis markers would be indicated in the same individuals, allowing a direct comparison and combination of different test results in a uniform research. In addition, because the serum CK-18 levels were measured qualitatively in a form of “increase” and “decrease”, or “positive” and “negative”, they failed to explore the quantitative data in this study. Further, this study collected baseline information at a single time point, without any follow-up inspection.
Taken together, our final results revealed that the serum levels of CK-18 were elevated in hepatitis patients, as compared to normal controls. The CK-18 levels could be adopted as a predictor for hepatitis progression. Further investigation is needed to determine the diagnostic mechanism and utility of apoptosis biomarkers, besides available blood test methods, for the detection of hepatitis.