1. Background
Hemangioma, a congenital vascular malformation, is the most common benign liver lesion with the prevalence of 3% to 20%, affecting females more than males. Liver hemangiomas usually are less than 5 cm in diameter and remain stable without any complications or malignant transformation subsequently no treatment is needed; however, complications comprising abdominal pain or fullness, coagulation disturbances, and inflammatory syndrome may occur (1, 2). In terms of diagnosis, hemangiomas have characteristic imaging features facilitating their diagnosis by modalities such as computed tomography (CT) scan, magnetic resonance imaging (MRI), and ultrasoungraphy (US) (2). Surgery is the first preferred option for treatment in case of need, including abdominal symptoms, complication such asKasabach-Merritt syndrome, jaundice, rapid growth, uncertain diagnosis, and Bornman syndrome (3). Interventional radiologists have used less invasive therapeutic modalities not only for the treatment of hepatobilliary diseases but also for other organs (4-9). Among these less invasive procedures; radio frequency ablation, radiation therapy, and trans-catheter arterial embolization (TAE) have been used for hemangioma. Of note, surgical management is not always attainable and has more complications in comparison to the other less invasive options (10, 11). TAE primarily has been performed as a pretreatment to shrink the tumor leading to the decrease in complications during surgery (12). Recently, it has been used as a single procedure for patients with liver hemangioma.
2. Objectives
In the present study, we aimed to assess the safety, feasibility and efficacy of TAE for the treatment of hepatic hemangioma using a retrospective review of 20 patients.
3. Patients and Methods
The present study was conducted in the department of diagnostic radiology of Imam Khomeini complex affiliated with Tehran University of Medical sciences, Tehran, Iran, from April 2013 to Jun 2014. Data of patients, who had hepatic hemangioma that was treated by TAE, were retrospectively reviewed. This study was approved by the Ethical Committee of Tehran University of Medical Sciences. Methods and procedures and their risks were explained in full detail to patients and all patients signed written informed consent before intervention.The diagnosis of hepatic hemangioma was made by imaging studies, including computed tomography (CT) scan, magnetic resonance imaging (MRI), and ultrasonography (US). Radiological features of hemangioam during TAE procedure confirmed the diagnosis. Routine blood tests, liver and renal function tests were obtained from all subjects before and after procedure.
3.1. TAE Procedure
TAE was performed by an interventional radiologist through gaining percutaneous trans-arterial access to the hepatic arteryby puncturing the common femoral artery under the general anesthesia and hepatic artery was catheterized. The embolic agent used was polyvinyl alcohol (PVA) particles (300-400 micron, Jonson and Johnson Cordis, USA).
3.2. Follow Up
All patients were followed up for 6 months. Imaging was carried out 6 months after TAE. Patients were also evaluated symptomatically through telephone interview by a physician.
3.3. Statistical Analysis
Statistical analysis was performed using SPSS software version 17 for comparison of tumor size before and after TAE by paired t-test. P Value less than 0.05 was considered statistically significant. Descriptive statistics used for patient’s characteristics expressed as mean and standard deviation (SD) for quantitative variables.
4. Results
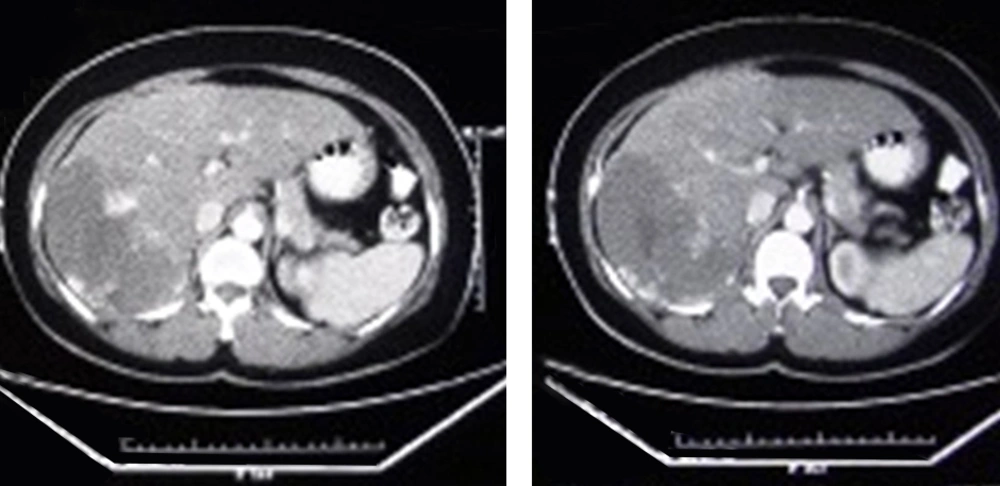
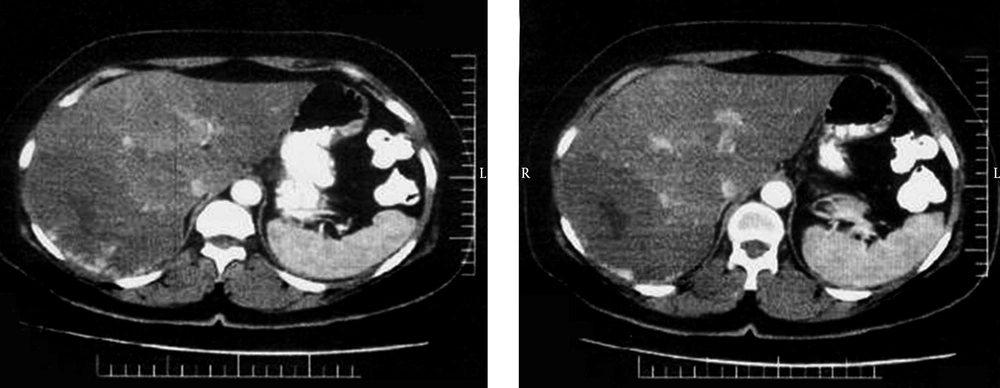
Twenty patients aged from 21 to 63 years (mean: 46.8, SD: 10.26) were included in this study. Clinical characteristic of patients are summarized in Table 1. Female patient represented the dominant population of the study. A total of 25 lesions were diagnosed. Five patients had 2 lesions. Indications for TAE were abdominal pain (n = 15) and rapid tumor enlargement (n = 5). TAE was performed on 20 lesions. Imaging modalities used in follow up were US and CT scan in 17 and 3 patients, respectively (Figures 1 and 2). Post embolization syndrome, including abdominal pain, fever, and leukocytosis occurred in one female patient 1 week after TAE and lasted for 3 days and was conservatively treated and she recovered within 3 days. No serious adverse event and TAE-related death was observed. None of the patient underwent another intervention including surgery. During follow up interval, decreased episode of abdominal pain was documented in all patients who had pain. Tumor enlargement was also stopped during follow up time. The average diameter of lesions that underwent TAE was 97.00 mm (range: 25-200 SD: 47.85) and 88.95 mm (range: 23-195 SD: 43.27) before and after embolization, respectively. Comparison of images before and after TAE revealed statistically significant decrease in the size of lesion (P Value: 0.004, t:3.31).
| No. | |
|---|---|
| Sex | |
| Female | 16 |
| Male | 4 |
| Number of lesions | 25 |
| Location | |
| Right lobe | 17 |
| Left lobe | 8 |
| Number of giant hemangimas, > 10 cm | 9 |
5. Discussion
Hepatic hemangiomas constitute the 70% of all benign liver tumors. The vast majority of these lesions are found incidentally by imaging studies and remain asymptomatic, therefore no specific treatment is required (1, 13). Nevertheless, complications such as rupture, bleeding, Kasabach-Merritt syndrome, and organ or vessel compression are the main indications for treatment of hepatic hemangiomas (3). Abdominal pain is a common symptom in patients while it has been stated that this pain is mostly ascribed to other gastrointestinal diseases such as irritable bowel syndrome. Therefore we should consider other causes in case of persistence of pain after treatment of hemangioma (14, 15). With regard to the size of the tumor, giant hemangiomas defined as larger than 4 cm in most of the studies while some authors considered giant hemangiomas as larger than 10 cm. According to the latter definition, giant tumors may contribute to produce events such as bleeding and rupture demanding a specific treatment. Of note, size of the tumor remains a controversial indication for therapy (3, 13). Surgical resection, preferably enucleation, is the first choice of treatment. Recently, other options have been applied for the treatment of hepatic hemangioma consisting of radiofrequency ablation, bevacizumab which is a monoclonal antibody, radiotherapy, chemotherapy, and TAE. Compared with aforementioned methods, surgery is a more invasive method and bears higher risks and longer term hospitalization (10, 11). Primarily, Yamamoto et al. have successfully performed TAE before surgical resection in a case of spontaneous rupture of a cavernous liver hemangioma (12). Accordingly, TAE was used as a method to shrink the tumor, particularly in giant hemangiomas, through blocking vascular reserve consequently leading to decreased hemorrhage during surgery (16). TAE has also been used effectively as a single therapy in recent studies in patients with hepatic hemangioma. However, there have been complications such as biliary tree damage (17), abscess formation, septicaemia, renal failure,bowel infarction,and post embolization syndrome (abdominal pain, nausea, fever, hypertension, thrombocytopenia, leukocytosis, and transient liver enzyme rise) (18). The preliminary goal of the present study was to ascertain the safety and feasibility of TAE. Our data showed that TAE is a safe and an attainable method for the treatment of hepatic hemangiomas. No major complication or any serious adverse event occurred during our study, except one patient who was a 28 year-old female that developed moderate post embolization syndrome. As a secondary goal, we aimed to assess the efficacy of TAE. In this regard, we compared follow up images with those taken before embolization as well as monitoring symptom relief in symptomatic patients. Our results showed that the size of tumors has been significantly decreased. In concerning of symptom relief, all patients who had abdominal pain or discomfort reported improvement or even some stated that the pain totally diminished. These outcomes are consistent with previous published studies (13, 19-25). Albeit, the great number of these studies have few sample sizes. In a study by Zeng et al. (24), 98 patients underwent TAE and followed up for 12 months. Imaging was done 6 and 12 months after TAE and compared with base line images revealing significant differences at both times. In contrast, Srivastava et al. (23) couldn’t show any significant changes in the tumor size after TAE performed on 8 patients. Although our data showed statistically significant decrease in tumor size, but it may not seem clinically considerable.In conclusion, our findings indicate that TAE is a safe and efficient procedure for the treatment of hepatic hemangiomas. A major limitation of our study was that we didn’t follow up patients for longer time, so further studies with larger sample sizes and long term follow up are required to support therapeutic effects of TAE.