1. Background
Salep or Lancibracteata is from orchid family (Orchidaceae) and has various species worldwide. This plant grows in the first days of summer and used for various attempt (1, 2). It is used in traditional medicine and food engineering. Nitrogenic materials, glucose, protein, ferolic acid, Quercetin, daucosterol, cirsiliol, steroids and glucomannan are some materials found in Salep (3, 4). This plant is used in preparation of drinks, confections and ice cream. On the other hand, it is used as a traditional medicine in various countries including Iran. In traditional medicine, it is used for the treatment of diarrhea, cough and impotency (5, 6). There is a belief in people that herbal drugs have no adverse effect. Nevertheless, we know that some herbal drugs may cause some adverse effects in some organs, especially liver and kidneys. A systematic review in Korea presented the possibility of increased risk of hepatotoxicity by administration of some herbal drugs (7). Despite the fact, some studies showed that some plants may have protective effect for liver such as Garlic in nonalchoholic steatohepatitis (NASH) (8), or Aubutilon indicum in paracetamol induced hepatotoxicity (9). Considering that Salep is widely used in some communities, evaluation of the effects of this plant on liver seems to be necessary.
2. Objectives
The aim of this study was to assess the effect of salep on liver for the first time.
3. Materials and Methods
3.1. Collection and Extraction of the Plant
Samples of the plant harvested from farmlands around Yasouj (a city in the southwest of Iran) were obtained. The roots of the plant washed and dried in the laboratory (away from direct light of sun). Then the dried roots ground into flour by electric mill. The powder mixed by ethylic alcohol in 1 to 5 proportions and mixed for 24 hours to yield a uniform solution. In the next stage, the solution filtered and dried for 48 hours to yield the solid extract without alcohol. The final extract mixed with distilled water in 20, 40 and 80 mg/mL and maintained in refrigerator (10).
3.2. Animals and Grouping Them
This study was an experimental study on Wistar rats. In this study, all the ethic points in working by animals were considered in all steps. The proposal of this study was approved in the research ethic committee of Jahrom University of Medical Sciences. In this study, 50 Wistar rats weighting 180 to 200 grams were used. Before starting the study, rats cared in animal house in Jahrom University of Medical Sciences for adaptation to environment. During the study, these animals were in 12 hours light and 12 hours darkness with temperature of 20-25°C. They had access to water and food ad libitum. We randomly divided the animals into five groups each with 10 rats. The first group underwent no intervention; the second group received 1 mL distilled water intraperitoneally daily, each rat in 3rd, 4th and 5th groups received 20, 40 and 80 mg/kg prepared hydroextract, respectively daily. This was continued for 29 days.
3.3. Evaluation of Liver Function Tests
In 29th day of study, blood tests for liver function were performed. For this purpose after anesthesia, 5 mL blood received from each rat and after centrifugation in 3000 r/m for 15 minutes serum was separated and used for tests (11). The levels of ALT (Alanine aminotransferase) and AST (Aspartate aminotransferase) were measured by DGKC buffer phosphate method and the level of ALP (Alkaline phosphatase) measured by ρ-Nitrophenyl phosphate AMP. Serum albumin and total bilirubin and total protein were measured by the methods of Bromocresol Green, Diazo with sulphanilic acid, and Biuret reaction end point, respectively. The level of MAD was evaluated by ELISA method (Biospes Italy) and the levels of TAC and TOC were also measured by ELISA (LDN Italy) (12).
3.4. Tissue Evaluation
After drawing the blood, liver was separated, weighted and fixed by formalin solution. Tissue samples were prepared and evaluated by microscope. Statistical analysis was performed using SPSS software (IBM) and P value below 0.05 considered to be significant.
4. Results
Liver enzymes evaluation; the levels of AST, ALT and ALP significantly decreased with administration of higher doses of salep (80 mg/kg). This effect was found in bilirubin MDA and TOC levels. On the other hand, it seems that with administration of salep the level of total serum protein and albumin elevated, especially with the highest dose. This effect was also shown in TAC level (Table 1).
| Parameter | Control | Distilled Water | Salep, 20 mg/kg | Salep, 40 mg/kg | Salep, 80 mg/kg |
|---|---|---|---|---|---|
| AST, IU/L | 93 ± 2.57 | 93.57 ± 2.30 | 87.71 ± 3.53 | 85 ± 2.94 | 76.14 ± 1.68 |
| ALT, IU/L | 47.85 ± 1.26 | 42.28 ± 0.81 | 45.57 ± 1.04 | 43.42 ± 0.87 | 39.85 ± 0.96 |
| ALP, IU/L | 485.42 ± 30.69 | 490 ± 29.27 | 432.57 ± 19.13 | 415.28 ± 15.29 | 343.71 ± 22.62 |
| Total protein, g/dL | 6.31 ± 0.06 | 6.41 ± 0.03 | 6.37 ± 0.10 | 6.3 ± 0.04 | 7.44 ± 0.06 |
| Albumin, g/dL | 3.18 ± 0.09 | 3.19 ± 0.07 | 3.25 ± 0.11 | 3.36 ± 0.10 | 4.04 ± 0.06 |
| Total bilirubin, Mg/dL | 1.01 ± 0.05 | 1.02 ± 0.04 | 0.97 ± 0.03 | 0.91 ± 0.04 | 0.88 ± 0.04 |
| TAC, U/mL | 1.5 ± 0.05 | 1.53 ± 0.08 | 1.74 ± 0.05 | 2.96 ± 0.10 | 3.56 ± 0.11 |
| TOC, U/mL | 2.36 ± 0.15 | 2.33 ± 0.15 | 2.16 ± 0.02 | 1.96 ± 0.03 | 1.8 ± 0.04 |
| MDA, Nmol/L | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.02 | 0.08 ± 0.01 | 0.05 ± 0.01 |
a Abbreviations: ALT, Alanine Transferase; ALP, Alkaline phosphatase; AST, Aspartate Transferase; g/dL, gram per deciliter; IU/L, Internation Unit Per Liter; MDA, Malondialdehyde; TAC, Total Antioxidation Capacity; and TOC, Total oxidation Capacity.
b P value below 0.05 was considered as significant. There was no significant statistical difference between the levels.
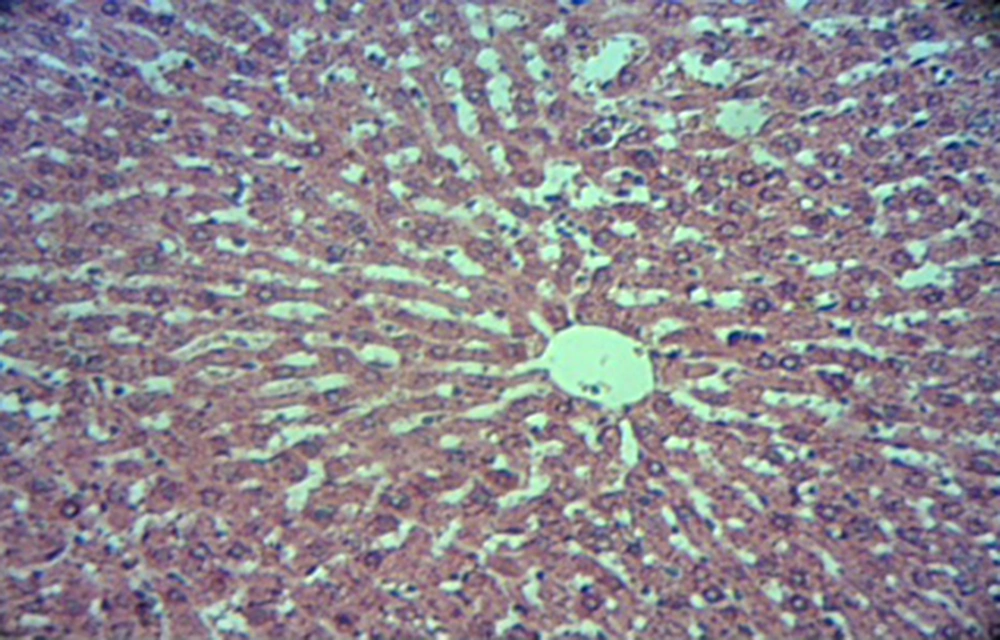
4.1. The Effect of Salep on Liver Tissue
Pathology of liver tissue evaluated and there was no sign of edema, irregularity of the hepatocells, vein engorgement, necrosis, accumulation of kupfer cells, change in portal area and infiltration of inflammatory cells. In short, no significant effect on liver tissue was revealed by various doses (20, 40, or 80 mg/kg) of salep (Figure 1).
5. Discussion
Injection of salep extract could decrease the level of liver function enzymes including AST, ALT and ALP. In liver damage, the level of these enzymes would be increased (13). This effect may be due to the presence of anti-oxidant material in this plant. Polyphenols and flavonoid components such as Quercetin are important anti-oxidants in this plant (14). These components have protective effect on the liver against toxins and free radicals (15, 16). Ferolic acid is another strong antioxidant in salep extract (3, 4). It is shown that ferolic acid has protective effect for liver against toxic materials such as alcohol and high fat foods. In these studies it was shown that ferolic acid can effectively reduce the level of ALT, AST and ALP in rats, which received alcohol and high fat foods (17). Glucomannan is a fiber, which is water soluble and can inhibit stress oxidation and reduce AST and ALT levels (18, 19). This component is present in salep 7% to 61% in various species. This fiber is also effective on reducing the blood sugar, cholesterol and body weight (20, 21). One of the signs of progression of chronic liver disease is decreased level of total protein and albumin. The level of this deduction is proportionate with the severity of liver damage (22, 23). In this study, we showed that the level of total protein and albumin could be elevated by salep. This elevation is significant with higher doses of salep. Therefore, we can say that this plant can have protective effect for liver. This effect is also shown for MAD, TOC and TAC in this study. MAD is one of the important criteria for lipid peroxidation and is an indicator for liver damage (24). TAC (Total Antioxidant Capacity) is a better criterion than GPX, CAT and SOD for evaluating the anti-oxidation condition in the body (25), this capacity has a reverse correlation with TOC (26). Augmentation of TAC and reduction of TOC and MAD by salep may be due to antioxidants in this plant. Polyphenols and flavonoids in this plant can protect cells against diminution of reduced glutathione. This action is performed by elevation of antioxidative enzymes such as glutathione, glutathione reductase, glutathione peroxidase and catalase (27). Reduced glutathione can diminish the oxidized form of glutathione peroxidase, which in turn can diminish hydrogen peroxide (H2O2) as a dangerous reactive component within the cell. Glucomannan can modulate the antioxidant system capacity. This component can elevate the level of SOD (supper oxide dismutase) and catalase enzymes. It also can decrease the MAD level in liver (28). In conclusion, salep may have a protective effect on liver in animal models and more studies are needed to evaluate its effect in human.