1. Background
Hepatocellular carcinoma (HCC), one of the major liver malignancies, ranks the fifth most common cancer and the third leading cause of cancer-related deaths worldwide (1). Each year, over 446000 cases are reported to newly occur in Asia and about 564000 all over the world (2). In particular, China, with a high prevalence of HCC, contributes to 55% of the world HCC cases (3). Persistent viral infections with either hepatitis B virus (HBV) or hepatitis C virus (HCV) are believed to be closely related to developing HCC, accounting for 53% and 25% of all HCC cases, respectively (4). Other high risk factors include cirrhosis, alcoholic liver disease, food aflatoxin intake etc. (5).
Currently, radiofrequency ablation (RFA), liver resection and transplantation provide the first-line effective treatment of HCC in early stage. Five-year survival rate of 70% was reported in HCC patients of single tumor less than 5 cm in diameter after surgical resections (6) and more than 70% in patients with HCC meeting the Milan criteria after liver transplantations (7). However, despite the relatively mature therapeutic methods, most HCC patients in China still present with advanced stage once diagnosed. For this reason, early detection of HCC appears increasingly important in China.
Alpha-fetoprotein (AFP) has long been used as a biomarker for HCC surveillance and an elevated serum concentration of AFP over 200 ng/mL is believed to have diagnostic significance (APASL guidelines) (8). However, it has been reported that some HCCs do not secret AFP (9). Besides, patients with cirrhosis and HCC can both be detected with an elevated level of AFP in sera (10). Thus, AFP as the only HCC biomarker needs to be improved.
Protein induced by vitamin K absence or antagonist-II (PIVKA-II), also known as Des-γ-carboxy-prothrombin (DCP), is another biomarker linked to HCC. Since first reported the notable association between HCC and an elevated serum level of PIVKA-II in 1984 (11), many studies focused on the use of PIVKA-II on HCC screening. The method for detecting PIVKA-II has been improved a lot, from previously the use of competitive radioimmunoassay with a polyclonal antibody (11) to currently chemiluminescence enzyme immunoassay (CLEIA) (12). Since it possesses relatively high sensitivity and specificity and most importantly, it is an independent factor for HCC surveillance (13, 14), a combination of PIVKA-II and AFP has been used for HCC screening for about two decades in Japan with satisfactory results (15, 16). Nonetheless, in China, whether PIVKA-II is an effective biomarker for HCC screening as AFP has not reached an agreement, and few reports published regarding sensitivity and specificity of PIVKA-II in the Chinese population.
2. Objectives
This study aimed to test whether PIVKA-II is an efficient biomarker specific for HCC and to identify the sensitivity and specificity of PIVKA-II alone or combined with AFP in Chinese patients with HCC.
3. Patients and Methods
A total of 481 patients with liver diseases (134 HCCs and 347 chronic hepatitis B patients without HCC) were included in this study. Besides, a total of 53 healthy volunteers and 105 patients with non-HCC cancers were tested as controls.
HCC was diagnosed using the following criteria recommended by Asian Pacific Association for the Study of the Liver (APASL) guidelines: 1) Typical HCC can be diagnosed by imaging regardless of the size if a typical vascular pattern, i.e. arterial enhancement with portal venous washout, is obtained on dynamic computed tomography (CT), dynamic magnetic resonance imaging (MRI) or contrast-enhanced ultrasound (CEUS). 2) Nodular lesions showing an atypical imaging pattern confirmed on further high resolution imaging systems, i.e. positron emission tomography (PET)-enhanced US (8).
The sera of patients with hepatitis B, with positive results for hepatitis B surface antigen (HBsAg) for at least one year, were obtained. Patients with cirrhosis and severe hepatitis, as well as the elders with positive HBeAg, were determined by either imaging screening or biochemical examinations. For healthy volunteers, they were enrolled from those of voluntary blood donation.
Samples meeting one of the following criteria were screened out: 1) Patients receiving warfarin or vitamin K before testing. 2) Samples contaminated or with precipitants or floccules. 3) Samples not enough for testing. All the sera were separated out and stored at-20℃ to ensure their freshness.
The study involved in the manuscript was approved by the ethics committee of Southwest Hospital, Chongqing, China. Informed consent was obtained from each patient included in the study and study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
3.1. Measurement of PIVKA-II and AFP
Serum concentrations of PIVKA-II were determined by CLEIA using monoclonal antibody to PIVKA-II (LUMIPULSE® G1200, FUJIREBIO INC., Japan) according to the manufacturer’s instructions. One arbitrary unit (1 AU) is of the same concentration as 1 mg of purified prothrombin. An elevation of over 40 mAU/mL is considered to be positive and abnormal.
Serum concentrations of AFP were measured by AFP Reagent kit with monoclonal antibody against AFP via Chemiluminescent Microparticle Immunoassay (CMIA) (ARTHITECT i2000, Abbott Laboratories, America). The cut-off for HCC is defined as 20 ng/mL.
3.2. Statistical Analysis
All the statistical analyses were performed using SPSS version 17.0 statistical software (IBM, USA) and the graphs were constructed on the GraphPad Prism version 5.02. The values of each biomarker are quantitative variables, which were represented as median with range and then transformed to logarithm form. Sensitivity, specificity, positive and negative predictive values, Kappa value and diagnostic accuracy were calculated in AFP and PIVKA-II both alone and combined by 2 × 2 table in SPSS. The combination was defined if either AFP or PIVKA-II had value greater than the cut-off. Spearman correlation test was applied to analyze the association between AFP and PIVKA-II in HCC detection. The Kolmogorov-Smirnov test was applied to check the normality of all quantitative data variables; it showed that values of each biomarker were skewed data. As a result, Mann-Whitney test was applied to compare the differences between two groups, and Kruskal-Wallis test for more than two groups. Pearson Chi-square test was applied to evaluate statistical differences between different types of liver diseases, different tumor sizes, different types of cancers and AFP and PIVKA-II on HCC screening. As for the diagnostic performance of AFP and PIVKA-II, receiver operating characteristic (ROC) curve analysis was applied for single marker and combination. Here, the combination of two biomarkers was obtained by binary logistic in SPSS. The area under the curve (AUC) and its 95% confidence interval (CI) were also performed automatically by SPSS. Differences or relationships were defined to be statistically significant when P value was below 0.05.
4. Results
4.1. Patient Characteristics
Of all the 134 patients diagnosed as HCC (mean age of 49.6 ± 11.9 (SD) years), a significant number of them (95.5%) were originally diagnosed and the remainders were relapsed after RFA. The male-to-female ratio was 8.6 (120 males and 14 females), with 21.6% of patients (n = 29) under 40 years old. In our series, most patients (90.6%) were developed from chronic hepatitis B. 85.8% (n = 115) of them had cirrhosis, with 95 patients were at uncompensatory stage. As for the controlled groups, patients with cirrhosis (n = 100), patients with severe hepatitis B (n = 94), patients with chronic hepatitis B (n = 103) and elders with positive HBeAg (n = 50) were randomly selected. Apart from these patients with hepatitis B diseases, non-HCC cancers were also selected as controls, including patients with lung carcinoma (n = 50), colorectal cancer (n = 25), prostate cancer (n = 12), esophagus cancer (n = 12) and gastric cancer (n = 6).
The size of the tumors in HCC patients varied a lot, but most were larger than 3 cm (73.9%) and over a half of them were present with huge size (> 10 cm, 53.3%). Of all the tumors, multiple genesis was in dominance (63.4%) and over a half of them presented in both lobes (54.5%). The basic characteristics of these patients are listed in Table 1.
| Variable | HCC (n = 134) | Liver Diseases (n = 347) | Other Cancers (n = 105) | Healthy Controls (n = 53) |
|---|---|---|---|---|
| Age, y | 49.6 ± 11.9 | 46.9 ± 9.8 | 57.7 ± 11.2 | 48.2 ± 11.8 |
| Gender | ||||
| Male | 89.6 | 70.1 | 78.1 | 58.5 |
| Female | 10.4 | 29.9 | 21.9 | 51.5 |
| Tumor number | NA* | NA | NA | |
| Single | 36.6 | |||
| Multiple (≥ 2) | 63.4 | |||
| Tumor size (n = 92) | NA | NA | NA | |
| < 3 cm | 24 (26.1) | |||
| 3 - 6 cm | 12 (13.0) | |||
| 6 - 10 cm | 7 (7.6) | |||
| >10 cm | 49 (53.3) | |||
| Localization | NA | NA | NA | |
| Right lobe | 36.5 | |||
| Left lobe | 9.0 | |||
| Both lobes | 54.5 | |||
| Etiology | NA | NA | NA | |
| HBV | 90.6 | |||
| HCV | 2.4 | |||
| Alcohol | 1.5 | |||
| Others | 5.5 | |||
| Portal vein invasion | 52 (38.8) | NA | NA | NA |
a Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; and HCV, hepatitis C virus.
b Values are presented as mean ± SD, % or No. (%).
4.2. PIVKA-II Levels Elevated in HCC Patients
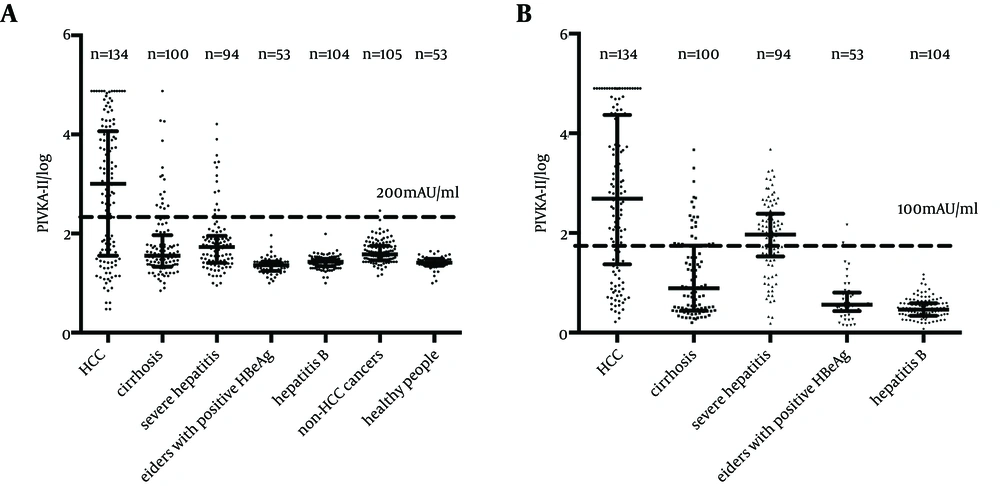
Serum levels of biomarkers in HCC patients and controlled groups are shown in Figure 1. PIVKA-II levels were significantly elevated compared with controlled groups. The median PIVKA-II levels in HCC, liver related diseases, non-HCC cancers and healthy people were 1012.0 mAU/mL (range: 1.0 - 75000.0 mAU/mL), 29.0 mAU/mL (range: 7.0 - 75000.0 mAU/mL), 38.0 mAU/mL (range: 14.0 - 291.0 mAU/mL) and 26.0 mAU/mL (range: 10.0 - 44.0 mAU/mL), respectively. P value was below 0.0001 comparing HCC with any of the other groups (by Kruskal-Wallis test). In all the controlled groups, serum levels of PIVKA-II in more than 95% of patients were below 200 mAU/mL, while in the HCC group, the serum level had a wide range with the mean level over 200 mAU/mL.
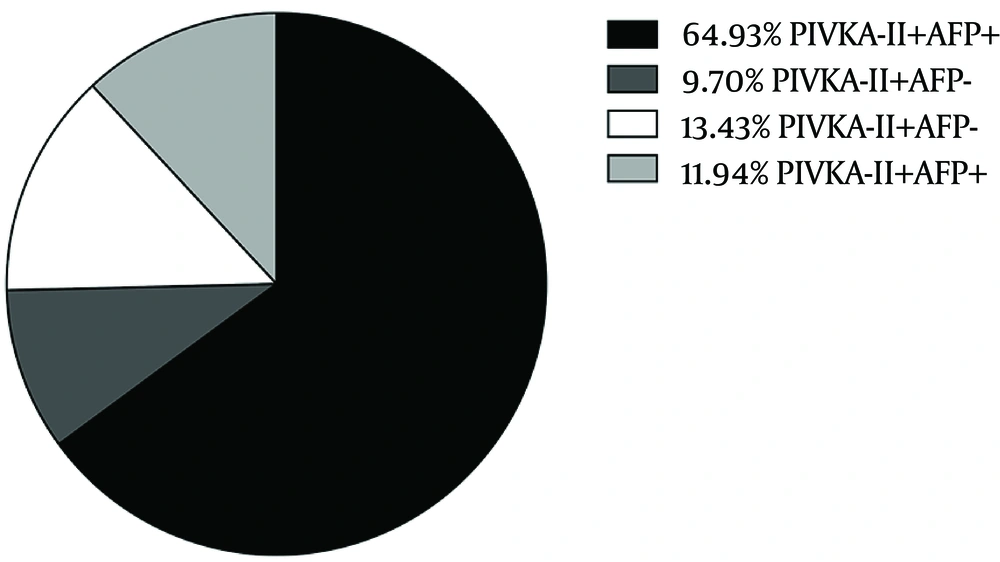
In this study, 40 mAU/mL was set as the cut-off value according to the manufacturer’s instructions based on previous studies. Under this level, 86.6% of patients with HCC were tested positive using the combined biomarkers, while for AFP alone, the positive ratio was 76.9%. (Figure 2) Compared with other non-HCC cancers, HCC groups showed a significant difference by Chi-square test (Pearson χ2 = 19.604, P < 0.001) for PIVKA-II. The same result also occurred in comparison between HCC group and non-HCC liver diseases. We noticed a relatively high positive ratio in severe hepatitis group both with AFP and PIVKA-II as biomarkers. However, Chi-square test found no difference between HCC group and severe hepatitis group with AFP (Pearson χ2 = 0.520, P = 0.471), but a significant difference with PIVKA-II (Pearson χ2 = 8.330, P = 0.004) (Figure 1 B).
4.3. Comparisons Between AFP and PIVKA-II Levels in HCC
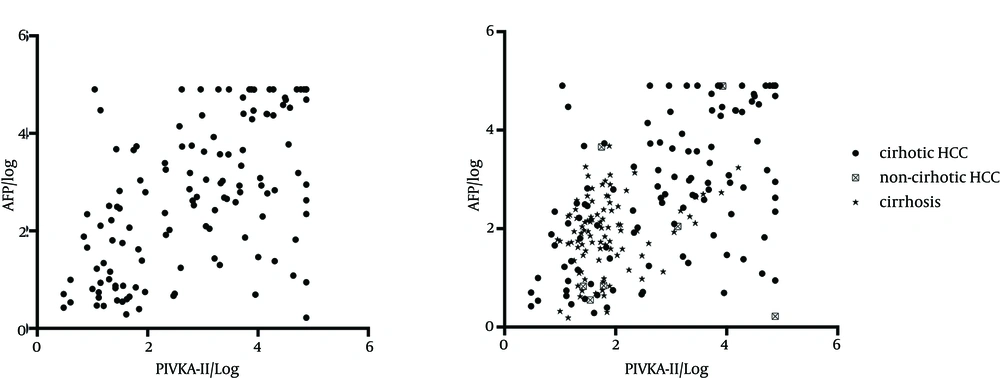
To figure out the association between AFP and PIVKA-II concentrations, the levels of PIVKA-II and AFP in HCC patients were shown in Figure 3 A after normalization using Spearman test (rs = 0.568, P < 0.001). With the cut-off value of 40 mAU/mL for PIVKA-II and 20 ng/mL for AFP, Chi-square test showed a significant difference between AFP and PIVKA-II (χ2 = 21.167, P < 0.001; Kappa = 0.397, P < 0.001). Compared with patients with hepatitis B diseases, the sensitivity, specificity, positive and negative prediction ratios and Kappa value were 74.6% (95% CI: 0.662 - 0.816), 68.6% (95% CI: 0.634 - 0.734), 47.8% (95% CI: 0.409 - 0.548), 87.5% (95% CI: 0.828 - 0.911), 0.369 (95% CI: 0.289 - 0.449) (P < 0.001) for PIVKA-II and 76.7% (95% CI: 0.684 - 0.834), 65.7% (95% CI: 0.604 - 0.706), 46.2% (95% CI: 0.395 - 0.530), 88.0% (95% CI: 0.833 - 0.916) and 0.352 (95% CI: 0.274 - 0.430) (P < 0.001) for AFP, respectively, while for the combination of the two markers, were 86.6% (95% CI: 0.793-0.916), 53.0% (95% CI: 0.476 - 0.584), 41.6% (95% CI: 0.358 - 0.476), 91.1% (95% CI: 0.861 - 0.945) and 0.297 (95% CI: 0.228 - 0.366) (P < 0.001), respectively (Table 2).
a Abbreviations: AFP, Alpha-fetoprotein; and PIVKA-II, Protein Induced by Vitamin K Absence or Antagonist-II.
b Patients with HBV-related diseases include patients with chronic HBV infection, severe hepatitis B, HBV-related cirrhosis and old carriers with HBeAg positive.
c PIVKA-II is measured in mAU/mL and AFP in ng/mL.
Different sizes of tumors showed significant difference in the sera concentrations of both AFP and PIVKA-II (Kruskal-Wallis χ2 = 52.473, P < 0.001) and the level elevated with the increase of size, but for the positive ratio of the same size between the two markers, PIVKA-II had no advantages (< 3 cm: 29.2% versus 62.5%; χ2 = 5.371, P = 0.02). The same phenomena also occurred for the number of tumors and different ages. Patients with HCC and cirrhosis displayed higher levels of both AFP and PIVKA-II than those with only cirrhosis (median AFP: 623.25 ng/mL versus 7.78 ng/mL, P < 0.001 by Mann–Whitney test; median PIVKA-II: 1580 mAU/mL versus 36 mAU/mL, P < 0.001 by Mann-Whitney test) (Figures 1 and 3 B). However, AFP levels in non-cirrhotic HCC patients showed no difference with patients with cirrhosis (Mann-Whitney U = 274.00, P = 0.338), while the level of PIVKA-II in non-cirrhotic HCC patients increased significantly (Mann-Whitney U = 184.00, P = 0.036). There was a trend toward higher levels of both AFP and PIVKA-II in HCC patients with portal vein invasion compared to non-invasion controls (median AFP: 1080.08 ng/mL versus 111.24 ng/mL, P < 0.001 by Mann-Whitney test; median PIVKA-II: 5407.5 mAU/mL versus 41.0 mAU/ml, P < 0.001 by Mann-Whitney test), but there was no difference in PIVKA-II between cirrhosis and non-invasion HCC and a significantly higher level of AFP in non-invasion HCC than cirrhosis.
Also in our study, the positive ratio had no difference either using AFP or PIVKA-II as a screening biomarker, when we compared them in the same subgroup as follows: the same tumor number, the same age group, HCC with or without portal vein invasion group and HCC with or without cirrhosis.
4.4. ROC Curve
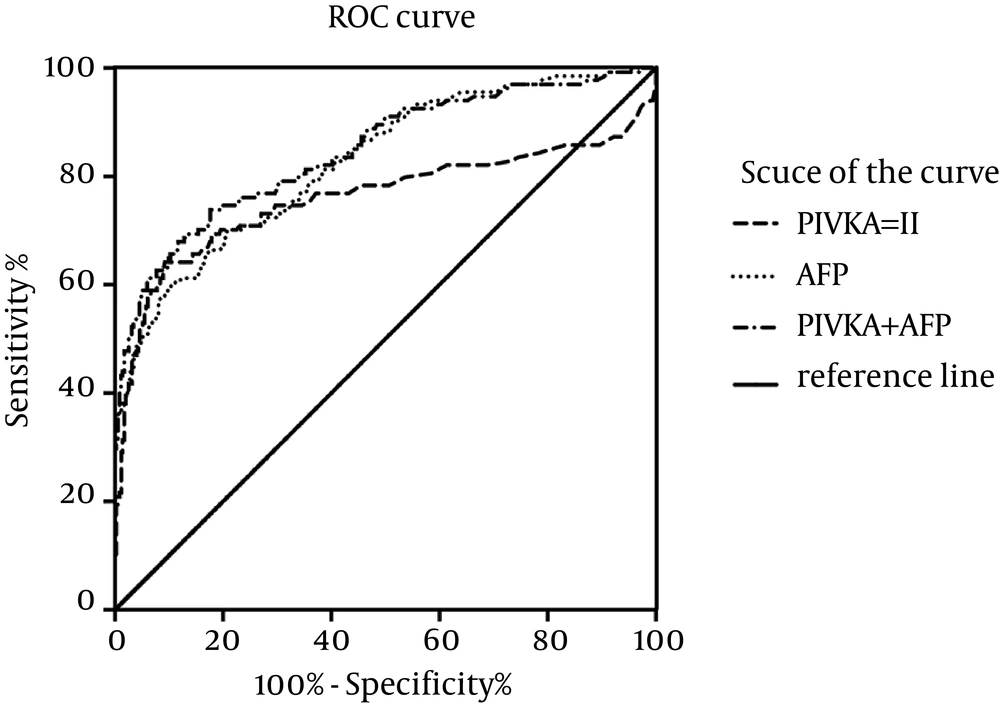
In our study, we depicted ROC curve to evaluate the diagnostic performance of PIVKA-II or the combination of AFP and PIVKA-II as biomarkers (Figure 4). For the combination procedure, two biomarkers were used as predictors and got every predicting possibility as the combining biomarker value using Logistic regression in SPSS binary logistic session. And then, ROC session in SPSS was applied to depict ROC curve. Finally, our curve showed that the area under the ROC curve (AUROC) for PIVKA-II (0.760, 95% confidence interval, CI: 0.699 - 0.820) was less than the AUROC for AFP (0.826, 95% CI: 0.784 - 0.869) in all HCC patients, but in combination, the AUROC could increase to 0.846 (95% CI: 0.804 - 0.888). Moreover, the best cut-off values indicated by our ROC curve were 195.2 ng/mL (Youden index: 0.501) for AFP and 200.0 mAU/mL (Youden index: 0.550) for PIVKA-II, respectively. Furthermore, these values corresponded to a sensitivity and specificity for PIVKA-II of 64.2% (95% CI: 0.554 - 0.721) and 90.8% (95% CI: 0.871 - 0.935), for AFP of 60.4% (95% CI: 0.516 - 0.687) and 89.6% (95% CI: 0.858 - 0.925) and for combination of 73.1% (95% CI: 0.647 - 0.802) and 83.3% (95% CI: 0.788 - 0.870), respectively (Table 2). Different sensitivities and specificities for different cut-off values of both AFP and PIVKA-II were given in Table 3.
The area under the ROC curve was shown with its 95% confidence intervals. AFP and PIVKA-II combined showed better performance than alone. The curves showed that the optimal cut-off value for PIVKA-II was 200.0 mAU/mL and that for AFP was 195.2 ng/mL. At the level of recommended cut-off values (40 mAU/mL for PIVKA-II and 20 ng/mL for AFP), the combination of the two markers had a sensitivity, specificity and Youden index of 86.6%, 53.0% and 0.396, respectively. And at the level of our new cut-off values, they were 73.1%, 83.3% and 0.564, respectively.
| Times of Cut-Off c | 1/2 | 1 | 2 | 2.5 | 5 | 10 | 25 |
|---|---|---|---|---|---|---|---|
| PIVKA-II | |||||||
| Sensitivity, % | 84.4 | 74.6 | 65.9 | 64.4 | 64.2 | 57.0 | 49.6 |
| Specificity, % | 21.4 | 68.6 | 84.0 | 85.2 | 90.8 | 92.9 | 94.9 |
| Youden index | 0.058 | 0.432 | 0.499 | 0.496 | 0.550 | 0.499 | 0.445 |
| AFP | |||||||
| Sensitivity, % | 80.7 | 76.1 | 72.6 | 67.4 | 66.7 | 60.0 | 48.9 |
| Specificity, % | 61.0 | 65.7 | 70.9 | 79.5 | 81.8 | 88.9 | 94.3 |
| Youden index | 0.417 | 0.418 | 0.435 | 0.469 | 0.485 | 0.489 | 0.432 |
a Abbreviations: AFP, Alpha-fetoprotein; and PIVKA-II, Protein Induced by Vitamin K Absence or Antagonist-II.
b The cut-off value for PIVKA-II is 40 mAU/mL and for AFP is 20 ng/mL.
c PIVKA-II is measured in mAU/mL and AFP in ng/mL.
5. Discussion
PIVKA-II being a good biomarker for HCC had nearly reached an agreement and is an effective biomarker for early HCC screening (15), besides, PIVKA-II is an independent biomarker for HCC screening. However, different researches have shown controversial results on whether PIVKA-II has better performances than AFP. Grazi et al. (17) indicated that the performance of PIVKA-II was lower than AFP; the AUROC of each marker was 0.812 and 0.887 (P < 0.0001), respectively. Marrero et al. (18) also reached the same conclusion. On the other hand, Volk et al. (19) showed that PIVKA-II had higher sensitivity and specificity than AFP. Li et al. (20), Sharma et al. (21) and Tateishi et al. (22) found the same result. However, Lok et al. (23) found that neither AFP nor PIVKA-II was an effective biomarker alone. Based on our study, PIVKA-II is just as potent as AFP if not more potent when used as a single marker, and in some aspects, AFP performed even better. By the commonly recommended cut-off values for AFP (20 ng/mL) and PIVKA-II (40 mAU/mL), AFP showed a specificity of 65.7% in our study, whereas PIVKA-II showed a slightly better specificity of 68.6%. Nevertheless, the AUROC and sensitivity of AFP were greater than PIVKA-II. Despite this, the combination of AFP and PIVKA-II presented great advantages as HCC screening biomarker with a maximum sensitivity of 86.6% and AUROC of 0.846. Thus, combination of AFP and PIVKA-II is recommended for HCC screening clinically. The same result was proved by Ertle et al. (24). As for cut-off values, different from what was currently recommended, we set 200 mAU/mL as our cut-off value. For one thing, serum levels of PIVKA-II in all our control groups were almost below 200.0 mAU/mL. For another, this was consistent with the cut-off value given by ROC curve. With new cut-off values (AFP: 195.2 ng/mL, PIVKA-II: 200.0 mAU/mL), PIVKA-II was much better than AFP in both sensitivity and specificity as well as Youden index. As a result, PIVKA-II was superior to AFP at our new cut-off values.
The controversial performance of AFP and PIVKA-II may be due to different causes of HCC. Since chronic hepatitis B was the pre-carcinoma disease in most of our HCC samples, it was reasonable that the sensitivity and specificity were different from the results above. Commonly, HCC samples were developed from hepatitis C and alcoholic steatohepatitis in western countries (25). Therefore, our results indicated that different types of HCCs could all be detected by measuring PIVKA-II level. Although most of our HCC patients developed from chronic hepatitis B, PIVKA-II was an effective HCC biomarker regardless of the etiology of HCC. That means PIVKA-II was applicable in Chinese population where most HCC patients evolved from chronic hepatitis B. Besides, most of our HCC samples were present with late stage and huge sizes, while small HCCs were relatively absent, thus the average PIVKA-II levels may quite vary.
In our analysis, AFP and PIVKA-II levels were evaluated in patients with severe hepatitis. But by the recommended cut-off values, AFP could not distinguish HCC from severe hepatitis, while PIVKA-II yielded a significant difference between these two. Moreover, AFP alone could not differentiate non-cirrhotic HCC from cirrhosis, but PIVKA-II could. In contrast, AFP had the capacity of distinguishing no hypovascular HCC from cirrhosis, but PIVKA-II did not. In our study, patients with non-HCC carcinomas were enrolled as controls. PIVKA-II level elevated in HCC group only, but its level did not elevate in patients with non-HCC cancers. These results indicated that PIVKA-II was a specific biomarker for HCC only. However, these two markers present non-specific elevation in some circumstances, such as severe hepatitis (AFP), warfarin treatment (PIVKA-II) and cirrhosis (AFP and PIVKA-II).
In conclusion, PIVKA-II was an independent biomarker for HCC screening and as effective as AFP, and thus may be applicable for HCC screening in Chinese population. But just like AFP, PIVKA-II may not be helpful for single use. The combination of AFP and PIVKA-II could increase sensitivity by 9.9% and positive rate by 9.7% compared with AFP alone. Further studies are needed to evaluate the efficacy of PIVKA-II on small (early stage) HCCs screening in Chinese population. In this case, perspective multi-center studies with large samples should be conducted to further confirm the screening performance of both PIVKA-II alone and AFP and PIVKA-II combination. It is also interesting to assess whether PIVKA-II is potent for screening non-HBV related HCC in Chinese population, such as alcoholic, HCV-related and autoimmune HCC.