1. Background
Infection with hepatitis C virus (HCV) is a grave public health problem and medical issue in the world. For years, the combination of pegylated interferon-alpha (PEG IFN-α) and ribavirin was the standard of care (SoC) for chronic hepatitis C (CHC) (1, 2). It is used to eradicate the infection, improve liver histology, and reduce risk of cirrhosis and hepatocellular carcinoma (3). This treatment regimen underwent a major change after the approval of direct antiviral agents (DAAs). DAAs inhibit specific steps in the HCV life cycle. Although triple therapy with DAAs increases sustained virological response (SVR) rate with decreased duration of therapy (4, 5), it brings significant adverse effects and increases the rate of becoming resistance variation. Furthermore, DAAs are expensive and are not yet available in many countries (6). The dual therapy as the classical approach is still the first line of treatment in China.
The commonly used endpoint for assessing efficacy is SVR, defined by the extinction of HCV-RNA six months after stopping treatment. Patients achieving SVR are considered to be virologically cured (7). However, a considerable number of patients fail to achieve SVR for viral breakthrough and unresponsiveness during treatment. In the follow-up of treatment with PEG IFN-α and ribavirin, some patients experience virological relapse after achievement of end-of-treatment response (ETR) even after SVR. Relapse, however, remains one of the leading issues in the treatment of patients with CHC. In order to reach SVR and avoid relapse, it is recommended to prolong the period of therapy for some “hard-to-treat” cases (8, 9). Long-term administration of PEG IFN-α/ribavirin increases the cost of treatment and the risk of serious adverse effects. Therefore, it is of great utility to effectively predict, either before or during treatment and follow-up, whether a patient will achieve an SVR and virus will be detectable again in patients. Several studies have defined the pretreatment patient features, such as viral genotypes and subjects with favorable IL28B genotypes, markedly affect the likelihood of attaining a SVR (1, 10-12). On-therapy kinetics of antiviral response also plays an important role; the rapid and early virological response are the most important factors (13-15). Moreover, although the relapse rate over time is one of the most important problems, which is clinicians’ concern, little is known about the effect of these values on relapse in the management of long-term follow-up.
2. Objectives
We have therefore conducted a retrospective and consecutive cohort study to assess the predictability of response to PEG IFN-α/ribavirin and relapse in patients with CHC. In addition, we retrospectively establish the prevalence of relapse among patients with ETR in the 24- to 288-week follow-up to provide clinical data to improve clinical outcomes.
3. Patients and Methods
3.1. Patients
Between February 2008 and March 2013, 169 previously untreated adult patients with CHC were consecutively treated with the combination of PEG IFN-α (2a or 2b) and ribavirin. They were followed up continuously before or after the end of treatment (at least 24 weeks) and nobody terminated treatment due to incompliance; therefore, the available data were comparable and complete. All of the patients enrolled at the Hepatology Outpatient Clinic of West China Hospital, Sichuan University, Chengdu, Sichuan, which is one of the largest governmental hospital in China. The diagnosis of CHC was based on serologic, biochemical, and virologic information according to the European association for the study of the liver (EASL) clinical practice guidelines: Management of hepatitis C infection, 2011. Exclusive criteria were as follows: co-infection with other hepatitis viruses or HIV; alcohol abuse (30 g/d); active intravenous drug use; severe hepatitis or cirrhosis; hepatocellular carcinoma; severe neuropsychiatric disorders; pregnancy or breast-feeding; poorly controlled thyroid disease or autoimmune diseases; uncontrolled depression; and hypersensitivity to Peg-IFN or ribavirin.
This study was approved by the ethics committee of West China Hospital of Sichuan University and each participant gave informed consent at data analysis according to the Helsinki declaration.
3.2. Laboratory Evaluation
Patients were tested for blood parameters, i.e., haemoglobin (Hb) level, white blood cell (WBC) count, and platelet (Plt) count, blood chemistry tests, ie, serum levels of creatinine (Cr), albumin (Alb), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), total cholesterol (Chol), and triglycerides (TG), thyroid function and autoimmune inhibitors at fourth and twelfth week, and then at three-month interval. In addition, they were assessed at fourth, 12th and 24th week and then every year after therapy. Eligible subjects were those with positive results for anti-HCV antibody in serum by chemiluminescence (Anti-HCV, Cobas, Roche Diagnostics GmbH, Germany). Serum HCV RNA was tested by quantitative and qualitative reverse transcriptase polymerase chain reaction (Ampliprep, Taqman, Roche Molecular Systems, Branchburg, the United States) with a lower limit of sensitivity of 100 viral copies/mL (50 IU/mL). HCV genotyping was done using reverse hybridization (INNO-LiPA, Innogenetics, Ghent, Belgium). All of the procedures were operated in strict accordance with the instructions. The IL28B SNP rs12979860 genotyping was detected by real-time allelic discrimination assay (TaqMan SNP Genotyping Assay, Applied Biosystems, Foster City, California, US) on a CFX96 Real-time PCR instrument (BioRad Laboratories, Hercules, California, US). The primers (Con F: GCTTATCGCATACGGCTAGG-3, Con R: CACAATTCCCACCACGAGAC-3) were included in 50 µL reaction volume designed by Invitrogen Biotech Company (Shanghai, China).
3.3. Study Design
This was a retrospective cohort study. All study patients underwent laboratory tests including determination of anti-HCV levels, HCV-RNA levels, HCV genotyping, IL28B SNP rs12979860 genotyping, determination of blood cell counts, serum ALT, determination of thyroid function including levels of thyroid stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4), determination of autoimmune inhibitors including autoantibody to nuclear antigen (ANA), and extractable nuclear antigen (ENA).
Patients were treated with 180 μg of PEG IFN-α-2a, or 1.5 μg/kg of PEG-IFN-α-2b subcutaneously once a week, while genotypes 1 and 6 received ribavirin at a dosage of 1000 to 1200 mg/d (based on body weight: 1000 mg/d for those ≤ 75 kg or 1200 mg/d for those > 75 kg) for 48 weeks and genotypes 2 and 3 received ribavirin at a dosage of 800 mg/d for 24 weeks. Dose modification or discontinuation of PEG-IFN and ribavirin were done at the discretion of each physician. All laboratory assessments were repeated at fourth and twelfth weeks, and then every three months of treatment. In addition, they were assessed in the serum at fourth, 12th, and 24th week and then every year after discontinuing the treatment. Grouping on treatment efficacy and relapse based on HCV RNA level.
3.4. Definitions
Rapid virological response (RVR) was defined as HCV RNA negativity at week four of treatment; early virological response (EVR) was defined as HCV RNA negativity at week 12 of treatment; ETR was defined as HCV RNA negativity at the end of treatment; SVR was defined as undetectable HCV RNA for 24 weeks after ETR. Non-responsive was defined as a patient who failed to achieve a decline of 2log10 HCV RNA IU/mL after 12 weeks of treatment or who never achieved undetectable HCV RNA during treatment duration of 24 weeks. Breakthrough (BT) was defined as reappearance of HCV RNA at any time during treatment after virological response. A relapse referred to detectable HCV RNA in serum after treatment discontinued and an ETR documented.
3.5. Clinical Follow-Up
Patients were asked to have follow-up of serologic, biochemical, and virologic test at fourth, 12th, and 24th week and then at least once a year after the treatment stopped. The period of follow-up ranged from six months to six years.
3.6. Statistical Analysis
Continuous variables across the treatment were provided as mean ± SD, and categorical variables as percentages. An analysis of variance was applied to continuous variables of the baseline factors in comparison to control groups and Chi square or Fisher’s exact tests were suitable for categorical variables. Efficacy of combination antiviral therapy (coded as 1, SVR; or 0, without SVR), relapse (coded as 1, relapse; or 0, without relapse) and non-response (coded as 1, non-response; or 0, response) were identified as dependent variable in binary logistic regression model. Several variables (age, sex, serum ALT, AST, and HCV RNA levels, HCV genotypes, IL28B rs12979860 genotype CC, and the presence or absence of RVR or EVR) were identified as independent variables. The results were presented as odds ratio (OR) and 95% CI. Cumulative incidence of relapse was plotted using the Kaplan-Meier method. All P values were two-tailed and statistically significance was accepted at P ≤ 0.05. Data analyses were performed using the SPSS 20.0 (SPSS Inc, Chicago, Illinois, US).
4. Results
4.1. Clinical Characteristics and Response Rates
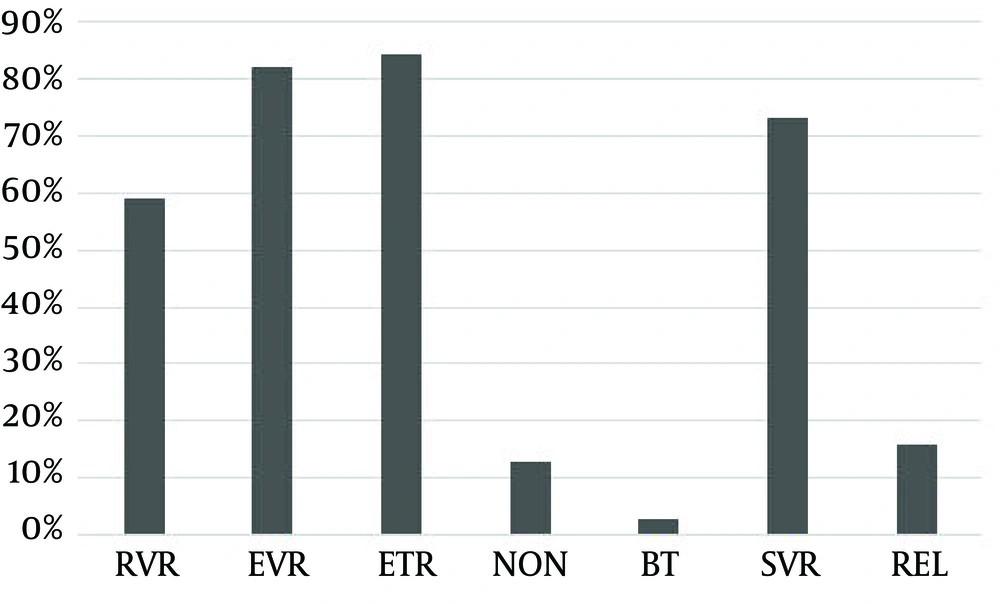
A total of 169 patients who had never received treatment were analyzed in this study. Patients received combination therapy with PEG IFN-α (2a or 2b) and ribavirin. The follow-up duration lasted at least 24 weeks and at most six years. Table 1 presents the results obtained from the preliminary analysis of demographics, biochemical, and virological data. For IL28B rs12979860, the frequencies of polymorphisms were 82.8% for the CC type and 17.2% for the CT/TT type. The response rate across the treatment at the end of the follow-up was as follows: 99 patients out of 169 (58.6%) had an RVR, 139 (82.2%) had an EVR, 142 (84.0%) achieved an ETR, 22 (13.0%) were non-response, 5 (3.0%) experienced viral breakthrough during treatment, 124 (73.4%) achieved an SVR, and relapses were observed in 23 patients after cessation of treatment. The prevalence of relapse in cases of ETR was 16.2% (Figure 1).
a Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; and IL28 CC, CC genotype of interleukin 28 single nucleotide polymorphism.
b Values are presented as mean ± SD or No. (%).
4.2. Predictive Factors Associated With Antiviral Combination Treatment
To explore the predictive factors based on antiviral combination treatment, we evaluated SVR according to demographic data and virology data as well as viral kinetics on-therapy. Demographic and virology data included age, sex, serum ALT, AST, and HCV RNA levels, IL28B genotypes, and HCV genotypes, and viral kinetics included the presence or absence of RVR or EVR (Table 2).
| Factor | SVR | Non-Response | Relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients With SVR (N = 124) | Patients Without SVR (N = 45) | P | Non-Response Patients (N = 22) | Response Patients (N = 147) | P | Patients With Relapse (N = 23) | Patients Without Relapse (N = 119) | P | |
| Age, y | 43 ± 13 | 44 ± 13 | 0.573 | 49 ± 11 | 42 ± 13 | 0.029 b | 43 ± 15 | 42 ± 13 | 0.736 |
| Sex (male: female) | 68 : 56 | 24 : 21 | 0.862 | 10 : 12 | 82 : 65 | 0.364 | 14 : 9 | 65 : 54 | 0.581 |
| ALT, IU/L | 76 ± 74 | 84 ± 72 | 0.460 | 71 ± 68 | 79 ± 74 | 0.615 | 80 ± 77 | 77 ± 75 | 0.333 |
| AST, IU/L | 66 ± 67 | 78 ± 67 | 0.226 | 67 ± 58 | 70 ± 68 | 0.870 | 75 ± 77 | 67 ± 67 | 0.435 |
| HCV-RNA, log10, IU/mL | 7.4 ± 1.6 | 8.8 ± 1.6 | 0.610 | 6.5 ± 0.7 | 6.1 ± 1.0 | 0.077 | 7.9 ± 1.5 | 7.5 ± 1.6 | 0.880 |
| HCV genotypes (1,6/2,3) | 90/34 | 43/2 | 0.001 b | 22/0 | 111/36 | 0.009 b | 20/3 | 88/31 | 0.181 |
| IL28B genotypes(CC/N-CC) | 112/12 | 28/17 | < 0.001 b | 13/9 | 127/20 | 0.002 b | 14/9 | 109/10 | < 0.001 b |
| RVR (RVR/N-RVR) | 89/35 | 10/35 | < 0.001 b | - | - | - | 8/15 | 87/32 | < 0.001 b |
| EVR (EVR/N-EVR) | 121/3 | 18/27 | < 0.001 b | - | - | - | 18/5 | 117/2 | < 0.001 b |
a Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CC genotype of interleukin 28 single nucleotide polymorphism; EVR, early virologic response; IL28 CC; SVR, sustained viral response; RVR, rapid virologic response.
b Statistically significance was presented.
We considered patients with SVR separately from those without SVR. Pretreatment factors and on-treatment virological response that might identify potential predictors of antiviral efficacy were compared between two groups. According to the results showed in Table 2, there were no significant differences in age, sex, and serum ALT, AST, and HCV RNA levels between groups. On univariate analysis, HCV genotypes (P = 0.001), IL28B genotypes (P < 0.001), RVR (P < 0.001), and EVR (P < 0.001) were associated with SVR. On multivariate analysis, AST level (OR = 0.993; 95% CI, 0.987 - 0.999; P = 0.032), IL28B genotype (CC: OR = 6.977; 95% CI, 2.111 - 23.057; P = 0.001), HCV genotype (OR = 7.057; 95% CI, 1.165 - 42.748; P = 0.033), RVR (OR = 3.018; 95% CI, 1.075 - 8.476; P = 0.036), and EVR (OR = 39.750; 95% CI, 8.561 - 184.565; P < 0.001) were independent predicting factors (Table 3). The positive predictive value of RVR was 89.9%, and that of EVR was 87.1%; there was no significant difference between them (P > 0.05). The negative predictive value of RVR was 50.0%, which was significantly lower than that of EVR (90.0%) according to Table 4 (χ2 = 14.261, P < 0.001).
| Factor | SVR | Non-Response | Relapse | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age | NA | NA | 1.050 (1.008 - 1.094) | 0.020 b | NA | NA |
| AST | 0.993 (0.987 - 0.999) | 0.032 b | NA | NA | NA | NA |
| IL28B genotypes (CC/N-CC) | 6.977 (2.111 - 23.057) | 0.001 b | 0.199 (0.069 - 0.577) | 0.003 b | 0.102 (0.031 - 0.339) | < 0.001 b |
| HCV genotypes (1,6/2,3) | 7.057 (1.165 - 42.748) | 0.033 b | NA | NA | NA | NA |
| RVR (RVR/N-RVR) | 3.018 (1.075 - 8.476) | 0.036 b | NA | NA | 0.239 (0.078 - 0.738) | 0.013 b |
| EVR (EVR/N-EVR) | 39.750 (8.561 - 184.565) | < 0.001 b | NA | NA | 0.102 (0.016 - 0.661) | 0.017 b |
a Abbreviation: AST, aspartate transaminase; EVR, early virologic response; IL28 CC, CC genotype of interleukin 28 single; OR, odds ratio; RVR, rapid virologic response; and NA, not applicable.
b Statistically significance was presented.
a Abbreviations: SVR, sustained virological response; EVR, early virologic response; and RVR, rapid virologic response.
b Statistically significance was presented.
A considerable number of patients failed to achieve SVR for never achieving undetectable HCV RNA levels in combination therapy. We separated responder from non-responder patients; the comparison results are shown in Tables 2 and 3. There are no significant differences in sex and serum ALT, AST, and HCV RNA levels between two groups. On univariate analysis, age (P = 0.029), HCV genotypes (P = 0.009), and IL28B genotypes (P = 0.002) were associated with non-responsiveness (Table 2). On multivariate analysis, old age (OR = 1.050; 95% CI, 1.008 - 1.094; P = 0.020) and IL28B genotype (N-CC: OR = 0.199; 95% CI, 0.069 - 0.577; P = 0.003) were independent risk predicting factors for non-responsiveness (Table 3).
4.3. Predictive Factors Associated With Relapse
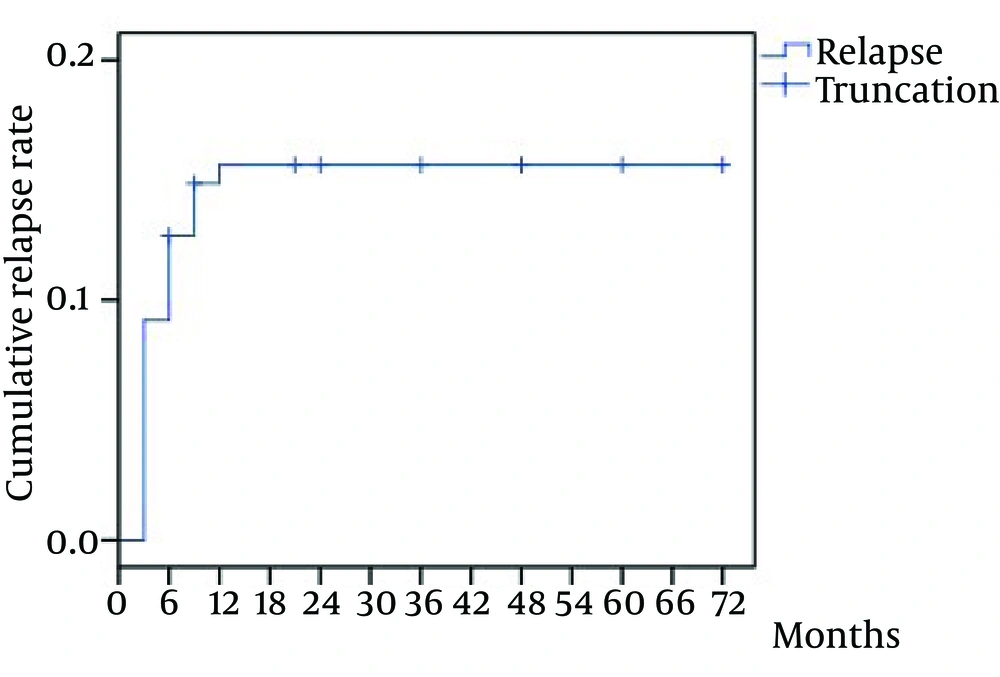
The median duration of follow-up was 22.93 months (range, 6 - 72 months). The overall rate of relapse was 13.6% (23/169) in the study cohort, while a recurrence of HCV RNA rate in cases of ETR was 16.20% (23/142). The earliest time of detectable HCV RNA was at the first month follow-up, the latest was at 48th month. Twenty-three patients had recurrence during follow-up 56.5% of which (13/23) relapsed between zero and 12 weeks after treatment, 21.7% (5/23) between weeks 12 and 24, and 17.4% (4/23) between 24 and 48 weeks. Otherwise, HCV RNA remained at undetectable levels at follow-up from two to six years, except in one patient (1/23) in whom it was detected at fourth year. The relapse rate within six months was significantly higher than other periods during six years of follow-up (χ2 = 7.792, P = 0.005). The cumulative rate of relapse increased more rapidly within six months and gradually slowed down in the subsequent period. The rate peaked at the 12th month follow-up and tended towards stability (Figure 2).
We categorized patients with relapse separately from those without relapse, the comparison results between the two groups are given in Tables 2 and 3. Baseline characteristics of patients were unrelated to a higher risk of relapse, except IL28B genotype (P < 0.001); meanwhile, the viral kinetics including RVR and EVR were related factors of relapse (both P < 0.001). Furthermore, we constructed a stepwise logistic regression analysis model to assess the impact factors of relapse rates. The model selected IL28B genotype (OR = 0.102; 95% CI, 0.031 - 0.339; P < 0.001), RVR (OR = 0.239; 95% CI, 0.078 - 0.738; P = 0.013), and EVR (OR = 0.102; 95% CI, 0.016 - 0.661; P = 0.017) as the negative predictor of relapse (Table 3). The positive predictive value of RVR and EVR were respectively 8.4% and 13.3% with no significant difference between them (P > 0.05). The negative predictive value of RVR was 68.1%, which was significantly higher than that of EVR (28.6%) (χ2 = 4.079, P = 0.043; Table 4).
5. Discussion
SVR and relapse maintain pivotal roles in the management of CHC infection. Thus, the prediction of achieving SVR and relapse are important to reduce adverse effects and therapy expenses. In China, PEG-IFN/ribavirin treatment, as the classical regimen, is still the first line of therapy; however, there was little data regarding the outcomes of long-term follow-up in CHC patients. In this study, we comprehensively explored the predictive factors of therapeutic effect and analyzed viral relapse during a six-month to six-year follow-up.
Non-responsiveness is an important component part of failing to achieve sustained viral response. Sezaki et al. (16) had explored response to treatment in patients with PEG-IFN/ribavirin in terms of age and sex. Their results showed male patients were more inclined to achieve SVR; regarding age, older patients had a lower tendency to achieve antivirus response. In the present study, the non-responsiveness was not associated with sex, but it was significantly associated with age. Therefore, age influences the severity of CHC. Older patients have faster disease progresses and poorer response to antiviral therapy. This phenomenon demonstrates the benefits of early intervention of treatment.
The SVR rates have been reported to vary depending on genotype. According to EASL guidelines, SVR rates are considerably higher in patients infected with HCV genotypes 2, 3, and 5 than in patients with genotype 1 (10). American Association for the Study of Liver Diseases (AASLD) guidelines indicate that SVR is achieved in 40% to 50% of patients infected with genotype 1 and in 80% or more patients infected with genotypes 2 and 3 (17). The SVR rates were 44% to 79% in Asian patients with genotype 1 and 75% to 94% in Asian patients with genotype 2 and 3 (18). These data suggest that genotype is a strong predictive factor of SVR.
In the present study, the overall SVR rate was 73.4% in all genotypes, 67.7% in genotype 1 and 6, and 94.4% in genotype 2 and 3. Patients with genotype 1 and 6 had significantly lower SVR rates compared to patients with genotype 2 and 3 (P = 0.001). Breaking the data down even further, we found no genotype 2 or 3 existing in non-responsive patients. It can further illustrate the role of HCV genotypes in combination treatment.
The heterogeneity in response to SoC treatment among different ethnic or racial groups can be partially explained by the finding that SVR rates are influenced by host genetic polymorphisms located upstream of the IL28B gene, which vary between different populations worldwide. We demonstrated that response rates and SVR rates were significantly higher in patients with IL28B rs12979860 genotype CC compared to patients with CT/TT (86.4% vs. 40.9% and 90.3% vs. 37.8%, respectively). This result was similar to that of De Nicola et al. (19) whose study showed that patients with CC genotype achieved significantly higher SVR rates compared to patients with CT/TT (88% vs. 38%, P < 0.001). Although the exact mechanisms behind this association are still only partially understood, there is unanimous agreement that IL28B genotypes were related to IFN-stimulated gene expression.
Therapeutic response can efficiently predict the outcome of therapy (20). Fried et al. (21) performed a retrospective analysis on 1383 patients and concluded that RVR was frequently an indication of EVR and could predict SVR (OR = 5.47; 95% CI, 3.97 - 7.52). Patients who become HCV-RNA negative after four and 12 weeks have a better chance of achieving an SVR. Our study confirmed that the patients who achieved RVR and EVR are more likely to have SVR than those who did not. This result was consistent with the previous reports (20, 21). Although we found that the positive predictive values of RVR were higher than those of EVR for all patients, which suggested that the earlier the patients gained virological response the more likely they gained SVR, there was no significant difference between them. This phenomenon demonstrates that the positive predictive values on SVR for RVR are similar to that of EVR. The negative predictive of RVR was relatively lower, while EVR had a stronger negative predictive value. It seems reasonable for those patients who did not achieve EVR to extend treatment for another 24 weeks (after treating for 12 weeks, HCV RNA is positive in serum, but drops ≥ 2 log10 in comparison to the pretreatment baseline) or stop treatment (up to week 12 of treatment patient do not achieve a 2 log10 drop in HCV RNA) to cease costs and adverse reactions, because there is slight chance of achieving SVR under this condition according to our study. It is also in agreement with EASL clinical practice guidelines. Moreover, stepwise logistic regression analysis about impact factors of SVR rate showed AST level as an emerging element (P = 0.032), which was not common in other studies. The AST level effect on SVR can be verified by further study with larger sample size.
It is encouraging to eradicate HCV-RNA during the antiviral therapy, but at the follow-up of treatment with PEG IFN-α and ribavirin, a considerable number of patients experience HCV recurrence after achievement of ETR even after SVR. Relapse, however, still remains a big problem for destroying confidence of patients with relapses and increasing the family and social economic burden. It is of great importance to analyze and predict relapses in the long-term follow-up. In our study, the prevalence of relapse in cases of ETR was 16.2%, which was consistent with previous report (22).
The duration of subsequent follow-up in these 169 patients ranged from six months to six years. The result of the present study shows that the highest percentage of relapses in patients who have received treatment with combination therapy occurs between week zero and week 24 of follow-up (78.3%) (P = 0.005). It indicates reinforced follow-ups are imperative within six months of stopping treatment. Nonetheless, about 17.4% relapses had undergone virological rebound after six-month follow-up, which suggests that patients yet to have a risk of relapse even after SVR and follow-ups are still required. In our study relapse was not observed after therapy was ceased for 48 weeks, except one patient (1/142) who experienced relapse at fourth year. The most likely explanation is reinfection rather than relapse. Therefore, we can conclude that HCV infection relapses are virtually non-existent in patients in whom HCV RNA is not detected after stopping therapy for 48 weeks.
The SNP rs12979860, upstream of IL28B gene, was associated with relapse in CHC treatment. The distribution between favorable allele (rs12979860 C allele) and unfavorable allele (rs12979860 T allele) is different in recurrence populations. The IL28B genotype was found to be highly predictive of relapse in this study. Moreover, our study indicated that individuals who achieved RVR/EVR had less chance to experience viral rebound. It suggested that the later the patients gained virological response, the more likely they experience relapse. Further analysis was performed to compare their predictive value. While the positive predictive values on relapse were similar in RVR and EVR, the negative predictive value of RVR was significantly higher than that of EVR. Although RVR had a stronger negative predictive value, the specificity is not high (68.1%). Some reported age as a significant risk factor for relapse. Older age was an independent risk factor for relapse; the older patients responded poorly to antiviral therapy compared with young ones (23). However, the significance of age has been reported with inconsistent results (24). In our study, age has not been observed as a hazard. This discrepancy could be due to weak power of significance and/or differences in sample size. Although the relapse rate of HCV genotypes 1 and 6 were higher than that of genotypes 2 and 3, there was no significant difference between them.
Limitations of our study were its retrospective nature, limited sample size, and lack of histologic examinations in all patients. Despite those limitations, SVR was significantly higher in patients with HCV genotypes 2 or 3, IL28B genotype CC, RVR, or EVR. Relapse during the first six months was significantly higher than other periods during six years of follow-up, but still about 17.4% patients with relapses experienced virological rebound between 24 and 48 weeks of treatment cessation. Patients might have a risk of relapse even after SVR and follow-ups are required. However, the relapse rate was significantly lower in patients with IL28B genotype CC, RVR, or EVR.
Our study clearly demonstrates the followings:
1) The SVR rate is related to HCV genotypes, IL28B genotypes, RVR, and EVR. Testing virus and host genotypes and utilizing the high sensitivity of RVR and the high specificity of EVR may be valuable to individualize the duration of therapy.
2) Virological rebound is mainly experienced during the first six months of treatment discontinuation, but quite a number of proportion relapse happened between 24 and 48 weeks of therapy cessation. We can conclude that relapse is virtually non-existent in patients after therapy cessation for 48 weeks, and therefore, follow-ups within the 48 weeks are actually imperative not in China. Relapse is predictable by IL28B genotypes, RVR, and EVR.