1. Background
Hepatitis B infection is caused by hepatitis B virus (HBV), a single, partially double-stranded DNA virus from the hepadnaviridae family. Worldwide, it is estimated that about 2 billion people are infected with HBV. Currently, more than 350 - 400 million are chronic carriers of the virus. HBV infection is one of the most common causes of hepatocellular carcinoma, which is among the top ten cancers worldwide (1-4). The safest and most effective method for the prevention of HBV infection is vaccination. Hepatitis B vaccine has been available since 1982. By the year 2008, more than 177 countries had implemented hepatitis B vaccination program (5). For a decade, vaccination strategies focused mainly on protection of individuals at high risks, but failed to reduce disease rates (6). The failure of such strategies led to the WHO recommendation that all countries should have either a universal infant or adolescent hepatitis B vaccination or both, integrated into their national immunization program by 1997 (7, 8).
Several previous studies from countries with varied HBV prevalence rates had shown that HBV vaccination in infancy induces protection for at least 10 - 15 years (5, 9-12). Vaccinees low or undetectable levels of anti-HBs antibodies are reported to develop a strong anamnestic response up to 10 years after primary immunization (13). Accordingly, several studies consider boosters to be unnecessary at 10 years after immunization (13-15). In 2000, the European Consensus Group and CDC issued a statement against the use of booster HBV vaccine in immunocompetent individuals 15 years after primary immunization unless accumulating data from different regions show a significant increase of HBV infection in adults vaccinated in childhood (12, 16, 17). However, other investigators continue to recommend booster dose because of the gradual decline of anti-HBs levels over time and potential risk of developing HBV infection (18).
Palestine, as other Middle Eastern countries, is considered an intermediate to high endemic area of HBV carriers (19). Starting from 1992, the Palestinian Ministry of Health implemented a national obligatory hepatitis B vaccination program for infants. Furthermore, in 1994, the vaccination program was expanded to cover all household contacts of HBV carriers and other high-risk groups such as health care workers, patients with multiple blood transfusion and others. However, after 10 - 13 years of implementing HBV vaccine program and despite the high coverage rate, the prevalence of HBV is still around 1.8% (20). During the years 2005 - 2011, the incidence rate of HBV ranged from 0.5 to 1.06 per 100,000. These data suggest a need to assess the efficacy of HBV vaccine after two decades of its implementation and check whether a booster dose is needed, especially in high risk groups.
2. Objectives
Therefore, the aim of the present study was to assess persistence of HBV surface anti-HBs response, as a reliable serology marker for vaccine-induced immunity among medical students at the Arab American University, Jenin, Palestine after 18 to 22 years of HBV vaccination.
3. Patients and Methods
3.1. Study Population
A cross-sectional study was conducted at the Arab American University in Jenin, West Bank, Palestine from September 2013 to April 2014. The study population included Palestinian students from the West Bank-Palestine and Palestinian students holding Israeli citizenship. Subjects were randomly selected from nursing, medical laboratory sciences and dentistry students. None of the students had participated in clinical training prior to or during the study. All students were born after 1992, and vaccinated at 0, 1 and 6 months of age according to the Ministry of Health recommendations using recombinant Engerix®-B (GlaxoSmithKline Inc.). Students were apparently healthy, with no acute or chronic illnesses. Ten milliliters venous blood sample was taken from each subject in a plain tube after signing a written informed consent. Serum was separated by centrifugation at 2000 × g for 5 minutes and aliquoted into two 1.5 mL micro-centrifuge tubes. Tubes were stored at -20°C until analysis. Each student was interviewed and a questionnaire including demographic, clinical history and health awareness parameters filled out. Data entered usingEpiInfo™7 free statistical software (Centers for Disease Control and Prevention (CDC) for analysis.
3.2. Laboratory Methods
All serum samples were tested for HBsAg, anti-HBc (IgG) and anti-HBs using the commercially available EIA kits (ELISA; Human Gesellschaft Fuer Biochemica und Diagnostica, Wiesbaden, Germany). Kits were used according to manufacturer’s instructions. All anti-HBs test results below 10 mIU/mL were repeated for confirmation. Anti-HBc positive samples were also repeated. Samples showing anti-HBs titer level below 10 IU/mL were considered non-protective, while those with anti-HBs titer more than 10 IU/mL were considered protective according to Jack et al. (21).
3.3. Molecular Assay
Viral HBV DNA was extracted from 200 µL of the 5 serum samples positive for anti-HBc using the QIAamp viral DNA extraction kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). The HBV DNA was amplified using nested PCR with two primer sets targeting the viral polymerase gene described previously by Selabe et al. (2007) using Thermo Scientific Reddy Mix (Thermo Fisher Scientific Inc.) (22). The PCR products from the second round were electrophoresed on 2% agarose gels and stained with ethidium bromide. The appearance of a 647-bp band was considered positive result. Negative control, nuclease-free water and a positive control were used in each run.
3.4. Health Knowledge
The students’ general knowledge and health awareness to hepatitis B infection was assessed by a questionnaire. The questions were about the cause of infection, symptoms, affected organ, mode of transmission and the best way to prevent the disease.
3.5. Statistical Analysis
EPi Info 7™ was used for descriptive analysis, frequencies and percentages. Association between variables and groups was calculated using Fisher’s exact and Chi-square tests. Correlation between variables was calculated using Pearson’s test assuming Gaussian distribution. P value < 0.05 was considered statistically significant.
4. Results
A total of 249 pre-clinical training students were selected for the study. The sample included 150 nurses (60%), 64 (26%) dentists and 35 (14%) medical laboratory technologists. Fifty six percent (140/249) were females and 44 % (109/249) males. They had a mean age of 19.8 ± 1.1 years, ranging from 18 to 22 years. All students started their compulsory basic/primary education at the age of 6 years. Around 58% (144/249) of Palestinian students were Palestinians holding Israeli citizenship and living in Haifa, Jaffa, Acre, Umm al-Fahm, the Triangle (Al Muthallath), Bir As-Saba’, Ar-Ramla, An-Nasira (Nazareth) and Bisan, while 42% (105/249) were Palestinians from the West Bank cities of Bethlehem, Al-Khalil (Hebron), Ramallah, Jenin, Tubas, Nablus, Salfit, Tulkarem and Jerusalem (Al-Quds). None of the students had positive results for HBsAg, while five (2%) had positive findings for anti-HBc. The five subjects with positive anti-HBc had negative results by PCR. In this study, 10 mIU/mL was considered as the cut-off value between immune and non-immune individuals. The overall rate of immunity (≥ 10 mIU/mL) was 75.5% (188/248). Table 1 shows lack of any statistically significant association between immune status and variables including age, sex, locality and level of anti-HBc.
| Immune | Non-Immune | P Valuea | |
|---|---|---|---|
| Gender | 0.30 | ||
| Male | 80 | 29 | |
| Female | 108 | 32 | |
| Locality | 0.13 | ||
| Palestinian | 75 | 30 | |
| Israeli Palestinian | 113 | 31 | |
| Age, y | 0.71 | ||
| 18 | 29 | 10 | |
| 19 | 43 | 19 | |
| 20 | 63 | 17 | |
| 21 | 45 | 12 | |
| 22 | 8 | 3 | |
| Anti-HBc | 0.24 | ||
| Positive | 5 | 0 | |
| Negative | 183 | 61 |
Distribution of Immune and Non-Immune Subjects by Sex, Locality, Age Group and Anti-HBc
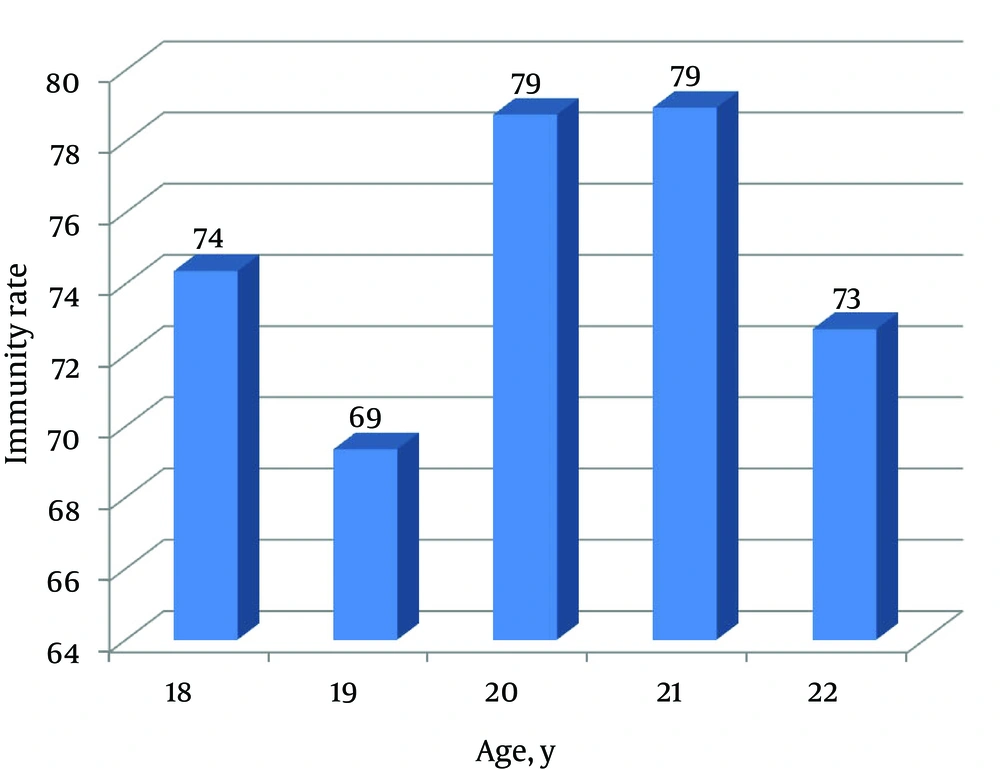
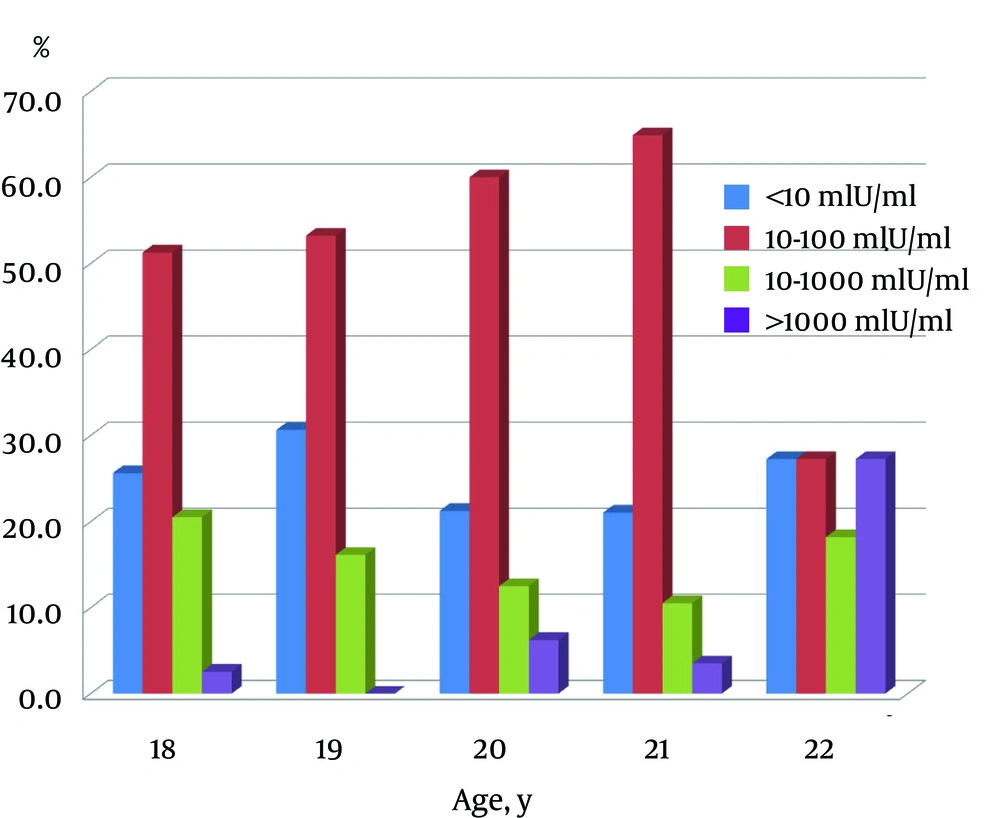
Correlation between immunity rate (students with titer > 10 mIU/mL in total students tested) and age was insignificant (r = 0.39, P = 0.53) (Figure 1). No correlation was found between age and anti-HBs concentration (r = 0.08, P = 0.20). About 57% of students had anti-HBs titer of 10 - 100 mIU/mL, 24.5% < 10 mIU/mL, 10% 100 - 1000 mIU/mL and only 4% above 1000 mIU/mL (Figure 2). The study group was well informed about hepatitis B disease for general public health knowledge as most were aware of causative agent (82%), main symptoms (72%), affected organ (89%), mode of transmission (81%) and prevention (82%).
5. Discussion
Medical Students in their first and second years are at high-risk of acquiring HBV infection through occupational exposure to blood and other body fluids during their clinical training in hospitals and medical facilities. Ideally, this group should be adequately assessed to ensure immunity against HBV infection. During the last two to three decades, a dramatic decrease in the prevalence of HBV infection had been achieved (23). This significant change in the prevalence is largely attributed to implementation of the obligatory HBV vaccine program at birth in more than 177 countries by 2008 (24). However, Zuckerman showed that HBV vaccine failure rates might reach up to 10% (25). Furthermore, several studies reported significant decline in anti-HBs levels with increasing age (26-28).
This study showed that most students (75.5%) still have immunity against HBV infection. These results are in agreement with those reported from China (79.5%), Thailand (60.5%), Saudi Arabia (60%) and the USA (66%) (28-31); nevertheless, higher than reported in Saudi Arabia (38%), Taiwan (53.5%) and Iran (48.6%) (27, 32, 33). Variation in the rate of anti-HBs persistence in previous studies, and even within the same country, may be largely attributed to differences in the type and dose of vaccines, age of initial vaccination, schedule of immunization, intervals between vaccine administrations, genetic pool of the target population, socio-economic status, compliance with the program and natural exposure to HBV infection.
The rate of immunity in this study group was not significantly affected by age, as the range was narrow enough, 18 - 22 years, not to show any statistical difference. Such results were also reported by Lu et al. (32) who showed similar protection rates in the age group of 15 to 18 years, while other studies showed that the protection rate significantly declines with increasing age from 10 to 24 years (26-28).
Five (2%) of the participants had anti-HBc, a marker of hepatitis B infection. However, all of them had negative results for both HBsAg by serology and HBV-DNA by PCR. Similar results had been reported from Taiwan (1.2%), Italy (0.4%) and the USA (1%) (5, 31, 34). The presence of anti-HBc indicates previous exposure to HBV infection at any point in life. Yet, simultaneous absence of HBsAg indicates that the vaccine has played its expected role in clearing the infection. Four of the 5 cases showed anti-HBs level in the range of 100 - 1000 IU/mL, while the fifth case had a titer of 13 IU/mL. This result indicates a strong immune memory response among the study participants and that waning or losing anti-HBs levels below 10 mIU/mL with age does not absolutely indicate loss of protection against HBV infection.
This study also revealed no difference in anti HBs protection rate between medical students with Israeli or Palestinian citizenship. Although both groups belong to two different political entities, they are from the same ethnicity and cultural background. Palestinian and Israeli health authorities are using the same type of vaccine, Engerix™-B and a similar immunization program (personal communication). Moreover, the Palestinian and Israeli childhood immunization coverage for HBV is similar, 99% and 96%, respectively (35, 36). The study also showed that, both male and female had an equal rate of protection. This finding was also reported by other studies in Italy, Saudi Arabia and Iran (5, 28, 37, 38). While, other studies from Saudi Arabia and the USA showed that females achieved a longer duration of protection (28, 31); these variations in anti-HBs protection level between male and female are still not well known and further investigation is needed.
This study demonstrated high level of health awareness towards HBV infection among study population. This finding is comparable to that of Noubiap et al. in Cameroon (39). In contrast, results from Syria and Laos showed that the first-year medical students had poor knowledge and lack of awareness about HBV disease, its routes of transmission, risk factors and modes of prevention (40, 41). The discrepancy could be due to variation in educational curricula prior and during university education.
The Centers for Disease Control and Prevention (CDC) recommends to perform annual testing when anti-HBs levels is below 10 IU/mL, and booster doses should be considered, especially among the high risk group (42). However, CDC, WHO and the European Consensus Group on Hepatitis B Immunity do not recommend booster dose in immunocompetent vaccinated individuals (43). In this study, 24.5% of medical students were non-immune and therefore should be considered for annual assessment and a booster injection. Previous studies on individuals vaccinated at birth aged 10 - 20 years, showed that booster vaccination of subjects with anti-HBs antibody titer below 10 mIU/mL was 93 - 100% effective (5, 30, 33).
In conclusion, this study demonstrated that students had good overall knowledge of HBV infection. Furthermore, the study showed persistence of anti-HBs antibodies in most of the study sample. Yet, one quarter of the students had a decreased level of anti-HBs (< 10 mIU/mL). The current strategy of the Palestinian and Israeli ministries of health is not to give a booster dose for medical students unless the anti-HBs level is below 10 IU/mL and HBsAg has negative result (Palestinian Ministry of Health, Personal communication and Israeli Ministry of Health websitehttp://www.health.gov.il). This complies with our results and should be encouraged.