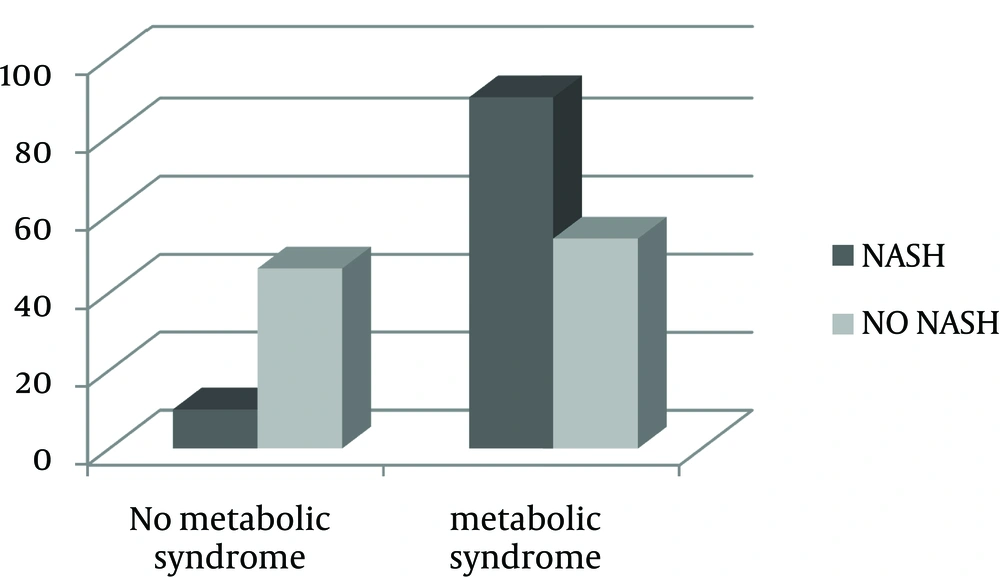1. Background
Obesity as a major worldwide health problem is associated with a group of chronic diseases including diabetes mellitus, cancer, cardiovascular disease (1-3) and non-alcoholic fatty liver disease (NAFLD), which is a broad spectrum from steatosis, non-alcoholic steatohepatitis (NASH) through cirrhosis and even hepatocellular carcinoma (HCC) (4). Obesity is the most common cause of NAFLD and NASH (5, 6) and NASH is known as the leading cause of cryptogenic cirrhosis (7).
Since severe liver injury may be present in asymptomatic obese patients and a definite diagnosis of NASH can only be made after an invasive procedure of liver biopsy associated with pain, high costs, pathologist-dependency and multiple risks, there is a need for noninvasive methods to predict the probability of NASH (8, 9). Several risk factors such as older age (10), high body mass index (BMI) (11), hypertension (12), insulin resistance (12), diabetes mellitus (13) or liver function tests abnormalities (14) have been considered as predictive factors for this serious liver disease.
On the other hand, many studies noted that vitamin D deficiency, increased serum parathyroid hormone (PTH) level and some other serum biomarkers are associated with obesity and some of its related comorbidities such as metabolic syndrome (15-18).
2. Objectives
The main aim of the present study was to investigate the possible role of vitamin D endocrine system in predicting the probability of presence of NASH in asymptomatic morbidly obese subjects selected for bariatric surgery in a surgery hospital in Iran.
3. Patients and Methods
Between December 2009 and March 2011, a total of 47 consecutive morbidly obese patients presented in our bariatric surgery center (Erfan hospital, Tehran, Iran) to undergo laparoscopic bariatric surgery. According to the national health institute’s guidelines, bariatric surgery criteria was defined as BMI > 40 kg/m2 or BMI > 35 kg/m2 with an obesity related comorbidity and failure to lose weight by nonsurgical treatments (19, 20). We excluded patients with previously proven hepatic diseases (hemochromatosis, Wilson’s disease, etc.), patients younger than 18 years old, consumers of more than 200 g of alcohol per week, subjects with positive viral markers (viral hepatitis or Human Immunodeficiency Virus) or positive antinuclear antibody (ANA), impaired renal function and those who receiving medications related to fatty liver disease, such as Tamoxifen, Valproate, Amiodarone, Diltiazem, Estrogens, corticosteroids and Methotrexate, or taking drugs affecting calcium or vitamin D metabolism such as phenytoin and corticosteroids, and recent consumption of calcium and vitamin D supplements (such as oral pills and injectable vitamin D3 and oral vitamin D2) during the past six months. Therefore, we enrolled 46 patients in our study and only one patient was excluded due to positive HBS antigen test.
3.1. Laboratory Testing
After recording anthropometric indices, blood pressure, baseline labs obtained including serum calcium (Ca), albumin (Alb), phosphorus (P), magnesium (Mg), Creatinine (Cr), alkaline phosphatase (AlP), 25-OH-Vitamin D3 (25 (OH) D), intact parathyroid hormone (PTH), fasting blood sugar (FBS), Hemoglobin A1c (HbA1c), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), total cholesterol (t-Chol) and uric acid. All blood chemistries were obtained in Erfan Hospital clinical laboratory by standard methods.
25 (OH) D and PTH levels were measured using ELISA method (Immunodiagnostic Systems (IDS) Ltd, Boldon, England). The normal range of 25 (OH) D is 30 - 150 ng/mL. 25 (OH) D concentration of 21 to 30 ng/mL is defined as vitamin D insufficiency. The normal range of PTH is 11 - 65.8 pg/mL (1.2 - 7 pmol/L). Vitamin D deficiency is defined as vitamin 25 (OH) D ≤ 20 ng/mL and also hyperparathyroidism is defined as PTH ≥ 65.8 pg/mL (7 pmol/L).
3.2. Study Design
Bariatric operations were performed by a single surgeon (K.T) at Erfan hospital, Tehran, Iran. During the laparoscopy operation, a wedge-shaped biopsy was taken from the left lateral segment of liver by the surgeon as a routine part of the procedure (21). All the biopsies were fixed and examined using Masson trichrome, silver reticulin and hematoxylin-eosin stain. A liver pathologist blinded to patients’ clinical condition and laboratory data, reviewed the histology slides and reported “having NASH” or “not-having NASH” using the following criteria for NASH definition: 1) lobular necroinflammatory foci, 2) ballooning degeneration of hepatocytes with or without Mallory bodies, 3) perisinusoidal fibrosis (22). Diagnosis of metabolic syndrome (MetS) was made according to the international diabetes federation (IDF) definition (Central obesity plus any two of the following four factors: triglycerides ≥ 150 mg/dL or specific treatment for this lipid abnormality, HDL < 40 mg/dL in males, HDL < 50 mg/dL in females or specific treatment for this lipid abnormality, systolic blood pressure (BP) ≥ 130 mm Hg or diastolic BP ≥ 85 mmHg or treatment of previously diagnosed hypertension, fasting plasma glucose ≥ 100 mg/dL or previously diagnosed T2DM) (23). According to the latest studies in Iran, waist circumference cut-off point for the diagnosis of MetS and central obesity in Iranian adults is 90 cm for both genders (24).
This study was performed in accordance with the 1964 Declaration of Helsinki on medical protocol and ethics and the ethical committee of Tehran university of medical sciences approved the study. All patients gave their written informed consent prior to enrolling in the study.
3.3. Statistical Analysis
Data are expressed as mean ± standard deviation unless otherwise indicated. The Kolmogorov-Smirnov test was applied to continuous variables to ensure a normal distribution of the variables. The significance of difference in continuous variables between groups was obtained by unpaired student’s t-tests and χ² test or Fisher’s exact test was used for discontinuous variables. Multiple logistic regressions were performed to assess odds for NASH. We fitted three models (NASH predicting models) for predicting the presence of NASH. Model-1: serum PTH and its known related parameters including Ca, Mg, Creatinine, 25 (OH) D and phosphorus considered as independent variables in a multivariate logistic regression that NASH (yes or no) was the dependent variable. Model-2: other possible confounding factors including age, gender, BMI, hypertension, current smoking, having diabetes mellitus type 2 (T2DM) and season of blood sampling were added to Model-1. Model-3: presence or absence of metabolic syndrome (MetS) was added to model-2 (15). Two-sided p value less than 0.05 considered as statistically significant for all tests. Data was analyzed using SPSS Statistics version 20 (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. Participants’ Characteristics
A total of forty-six obese subjects (females: 34 [74%]; males: 12 [26%]) with a mean age of 36.5 ± 10.6 years (ranged 18-54 years) and a mean BMI of 45 ± 7.3 kg/m2 included in this study. All patients were Iranian. Twenty (43.5%) of the patients had NASH and the rest of 26 patients (56.5%) did not fulfill the histological criteria for NASH. The prevalence of 25 (OH) D deficiency (≤ 20 ng/mL) and hyperparathyroidism (≥ 65.8 pg/mL) were 78.3% (36 patients) and 32.6% (15 patients), respectively. Thirty-two (69.6%) of patients fulfilled the criteria of having metabolic syndrome and all patients had serum Ca and P in normal ranges.
4.2. Characteristics According to Absence or Presence of NASH
Biochemical and clinical characteristics of obese patients are shown in Table 1. Age, gender and BMI were not significantly different between groups. Although 25 (OH) D levels were low in both groups, the mean did not differ significantly. Whereas, PTH, AST and ALT levels were significantly higher in the group with NASH (P value < 0.001 for PTH and ALT, and < 0.01 for AST). Frequency of MetS was significantly higher in the NASH group (90% vs. 53.8%) (Figure 1).
| Patients Without Non-Alcoholic Steatohepatitis (n = 26) | Patients With Non-Alcoholic Steatohepatitis (n = 20) | P Value | |
|---|---|---|---|
| Gender | 0.227 | ||
| Female | 81 | 65 | |
| Male | 19 | 35 | |
| Age, y | 35.5 ± 9.43 | 39.0 ± 10.1 | 0.233 |
| BMI, kg/m2 | 44.3 ± 5.82 | 45.2 ± 8.99 | 0.704 |
| AST, U/L | 20 ± 9.68 | 32.3 ± 17.48 | 0.009 |
| ALT, U/L | 24.2 ± 13.5 | 44.2 ± 24.8 | 0.003 |
| AST/ALT | 0.88 ± 0.20 | 0.76 ± 0.16 | 0.028 |
| VitD3, ng/mL | 14.1 ± 13.5 | 8.71 ± 5.58 | 0.126 |
| PTH, pg/mL | 40.4 ± 19.7 | 84.5 ± 48.5 | < 0.001 |
| Ca, mg/dL | 9.14 ± 0.49 | 9.20 ± 0.42 | 0.688 |
| P, mg/dL | 3.36 ± 0.53 | 3.20 ± 0.56 | 0.359 |
| Mg, mg/dL | 2.12 ± 0.21 | 2.13 ± 0.23 | 0.886 |
| Alb, g/dL | 4.36 ± 0.52 | 4.38 ± 0.39 | 0.887 |
| FBS, mg/dL | 108.2 ± 25.9 | 109.8 ± 25.2 | 0.837 |
| HbA1c, % | 5.89 ± 0.91 | 6.22 ± 1.08 | 0.270 |
| T-Chol, mg/dL | 192.4 ± 36.4 | 182.4 ± 32.0 | 0.344 |
| LDL, mg/dL | 110.8 ± 26.4 | 107.0 ± 23.0 | 0.613 |
| TG, mg/dL | 151.0 ± 82.7 | 158.7 ± 58.0 | 0.729 |
| Alp, IU/L | 230.7 ± 183.3 | 193.1 ± 63.5 | 0.397 |
| MetS, % | 53.8 | 90 | 0.008 |
4.3. PTH, 25 (OH) D and Odds for Having NASH
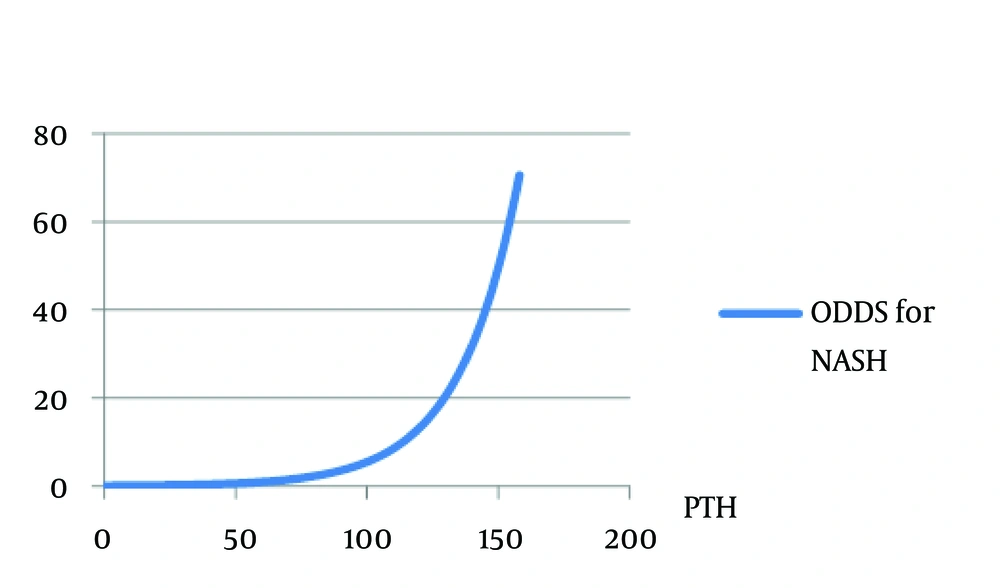
PTH was significantly correlated with 25 (OH) D (r = -0.308, P value: 0.04). In unadjusted logistic model, PTH was the only variable in vitamin D endocrine system (Ca, P, 25 (OH) D, PTH), which was significantly associated with NASH (odds ratio (OR): 1.0445, 95% CI: 1.015 - 1.075, Nagelkerke’s R2 = 0.403, χ² = 8.765, P value: 0.003) in our morbidly obese patients (Figure 2). In NASH predicting logistic models introduced previously in methods, PTH was significantly associated with having NASH in all the three models (Table 2). In the second and third models, we can also see the age as the variable correlated with presence of NASH.
| Model-1 | Model-2 | Model-3 | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P Value | OR (95%CI) | P Value | OR (95%CI) | P Value | |
| PTH | 1.09 (1.01 - 1.17) | 0.021 | 1.08 (1.01 - 1.16) | 0.027 | 1.08 (1.01 - 1.16) | 0.030 |
| VitD3 | 0.98 (0.9 - 1.1) | 0.72 | 0.98 (0.9 - 1.1) | 0.696 | 0.97 (0.9- 1.1) | 0.641 |
| Age | - | - | 1.22 (1.01 - 1.5) | 0.039 | 1.22 (1.0 - 1.5) | 0.043 |
a Abbreviations: CI, confidence interval; OR, odds ratio.
To complete our work, we assessed quartiles of 25 (OH) D and PTH as a dichotomous variable (hyperparathyroidism vs. normal PTH) instead of continuous variable and performed logistic regression for this new categorical independent variable; we again found no significant association between 25 (OH) D quartiles and presence of NASH in obese patients, but the model of hyperparathyroidism and NASH showed significant association between these two new variables (Table 3) (odds ratio (OR): 6.722, Nagelkerke’s R2 = 0.220, χ² = 7.298, P value = 0.007).
| Patients | Parathyroid Hormone | |
|---|---|---|
| Normal | Elevated | |
| Patients without non-alcoholic steatohepatitis | 47.8 | 8.7 |
| Patients with non-alcoholic steatohepatitis | 19.6 | 23.9 |
a Data are presented as %.
4.4. Metabolic Syndrome and Presence of NASH
In binary logistic regression model, in which metabolic syndrome (presence/absence) was introduced as an independent variable, metabolic syndrome was found to be a predictive factor for NASH (odds ratio: 7.714, 95% CI: 1.48 - 40.2, Nagelkerke’s R2 = 0.205, χ² = 5.88, P value = 0.015) (Figure 1), but after adjustment for PTH levels, this interaction did not remain significant anymore (P value = 0.112, CI: 0.713 - 25.54).
4.5. AST, ALT and NASH
Using logistic regression, when AST, ALT and AST: ALT ratio were considered separately as independent variables to predict NASH, a significant correlation was found between each of these variables with NASH (OR: 1.08 [95%CI:1.015 - 1.146], 1.06 [95%CI:1.015 - 1.102], 0.022 [95%CI:0.001 - 0.854], respectively.
4.6. Age at Liver Biopsy and NASH
Age at liver biopsy and NASH did not show any correlation when it was considered the only independent variable, but after adjusting for other factors including PTH, sex, BMI, season of blood sampling, current smoking, MetS, Ca, Mg, P and vitD3, age showed its correlation with NASH (Table 2).
5. Discussion
The novel finding of this study was elevated serum PTH level as the predictive factor for NASH in morbidly obese patients seeking bariatric surgery, independent of other factors introduced previously.
5.1. Prevalence of NASH
In our study, 43.5% of forty-six morbidly obese patients, candidates for bariatric surgery met the criteria for NASH. Because of a number of factors such as silent nature of NAFLD and its important subgroup, NASH, reports about the prevalence of this disease in obese patients are quite variable. However, our findings are consistent with a study by Spaulding et al. (25) and Ong et al. (26), in which prevalence of NASH were 56% and 23.5% respectively, in morbidly obese patients. Although the difference was not significant, in our study this prevalence in men and women were 58.3% and 38.2% respectively, which is consistent with the study by Arun et al. (27), in which 60.3% of morbidly obese men and 30.9% of obese women were diagnosed with NASH.
5.2. PTH, 25 (OH) D and NASH
This study demonstrated that PTH but not 25 (OH) D was associated significantly with nonalcoholic steatohepatitis in morbidly obese patients. Although, several lines of evidence have already discussed the impact of vitamin D-endocrine system on obesity and its related comorbidities such as metabolic syndrome, there are very few published studies about the effect of this system on liver histopathology in obese subjects. Hjelmesaeth et al. (15) in a cohort study of 1017 morbidly obese patients reported that PTH is an independent predictor of metabolic syndrome in morbidly obese patients, whereas there is no association between MetS and 25 (OH) D. However, this finding was not confirmed by some other studies (28, 29). Targher et al. (30) found that NAFLD patients have a significant decrease in serum 25 (OH) D levels, which is inversely associated with severity of liver histopathology (subjects were not selected from morbidly obese patients in this study).
Interestingly, despite PTH effects on bone and kidney, some hypotheses indicate that excess PTH may promote weight gain with increasing intra-adipocytes free calcium, thus blunting the lipolytic response to catecholamines (31).
5.3. Liver Enzymes and NASH
In our study, the mean level of both AST and ALT were higher in obese patients who met the histological criteria for NASH. Many previous studies also confirmed this finding and introduced elevated liver enzymes as the predictor of NASH (10, 14). Because liver enzymes might be in the normal range in patients with NASH, these two tests are not good predictors of this disease.
5.4. Age and NASH
Because of the potential association of age with other biological factors, we could not find the effect of age on presence of NASH at first, but after adjusting for other variables, an association found between age and NASH. Thus, as a morbidly obese patient gets older, the probability of progression to NASH would be increased. This finding is consistent with previous studies which introduced increasing age as a risk factor for NASH (10, 13).
Recent consumption of calcium and vitamin D supplements can affect serum PTH and 25 (OH) D levels, and it could be a limitation for determining the role of vitamin D endocrine system in predicting the probability of presence of NASH. We excluded patients with recent consumption of calcium or vitamin D supplements from the study to overcome this limitation.
In conclusion, the present study found elevated serum liver enzymes, high serum PTH levels and older age as predictors of NASH in morbidly obese patients seeking obesity surgical treatments. If studies with large sample sizes from different ethnic groups confirm our findings, serum PTH level may help clinicians to screen patients with NASH. However, liver biopsy is still the gold standard for detecting NASH.