1. Background
Hepatitis C virus (HCV) is an important human pathogen affecting an estimated 120 – 170 million individuals throughout different regions of the world. This virus can cause acute and chronic hepatitis, liver cirrhosis and hepatocellular carcinoma (1). This virus is an enveloped positive strand RNA virus, a family member of Flaviviridae, with a genome size of 9.6 kilobases, composed of structural (core, envelope 1 and 2) and nonstructural (NS) proteins 2 - 5 (2, 3). The virus was recognized in 1989 as the infectious agent responsible for the majority of transfusion-associated non-A, and non-B hepatitis (4). Hepatitis C virus encodes a single polyprotein, which is then processed into 10 mature structural and regulatory proteins. Furthermore, HCV proteins and HCV RNA can induce a host immune response. Although antiviral therapies have been improved considerably in the recent years, no effective vaccine has been developed thus far. The standard-of-care treatment for patients, includes combination of Pegylated Interferon (PEG IFN)-α and ribavirin, which is associated with side effects (5). Toll-Like Receptors (TLRs) are pattern-recognition receptors that were discovered in the mid-1990s. Up to now, 10 and 12 functional TLRs have been identified in humans and mice, respectively, which recognize Pathogen-Associated Molecular Patterns (PAMPs), and induce immune responses (6, 7). Toll-Like Receptors are largely divided to two subgroups according to respective PAMP ligands and their cellular localization. One group is composed of TLR1, TLR2, TLR4, TLR5, TLR6 and TLR11, which recognize components such as lipids, lipoproteins, and proteins, and are expressed on cell surfaces. Another group is composed of TLR3, TLR7, TLR8 and TLR9, which are expressed in intracellular vesicles such as the endoplasmic reticulum, endosomes, lysosomes, and endolysosomes. The latter group recognizes microbial nucleic acids (6). When TLRs bind to their appropriate ligand, upregulation of pro-inflammatory cytokine and chemokine production and interferon signaling initiates by a downstream signaling cascade. Toll-Like Receptors provide a bridge between innate and adaptive immunity. Immune responses are critically important in viral infections, including HCV infection (8, 9). The TLR1, TLR2 and TLR6 act as pattern receptors for HCV core and NS3 mediating myeloid differentiation primary response gene 88 (MyD88) activation, which leads to nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ϰB) to arbitrate inflammatory cytokine response as well as nitric oxide production (10). As HCV is a single-stranded RNA (ss-RNA) virus, it is supposed to be susceptible to detection by TLR7 and TLR8 (11).
Various studies have been conducted on TLRs mRNA expression level in HCV infection and they have shown different results for TLR expression at different stages of infection. The study of Wang et al. indicated an increase in TLR2 and TLR4 mRNA expression levels during HCV infection (12), while Dolganiuc et al. showed that RNA levels for TLR 2, TLR6, TLR7, TLR8, TLR9 and TLR10 mRNA were up regulated in HCV-infected patients compared to controls (13). Atencia et al. showed that TLR3 and TLR7 mRNA levels are significantly down-regulated in patients with chronic HCV infection when compared with healthy controls (14). However, there are controversial results about the mRNA expression level and the role of TLRs in patients with HCV infection.
Many studies have supported the involvement of TLR2 in HCV core and NS3 mediated inflammation (15, 16). Also HCV, as an ss-RNA virus, is supposed to be susceptible to detection by TLR7. TLR7 is of particular interest from a medicinal chemistry standpoint because small molecule ligands for this receptor have been identified, including the guanosine analog isatoribine .Statistically significant antiviral effect with relatively few and mild side effects for TLR7 agonist (isatoribine) is expressed (17). Therefore, in the present study we focused on TLR2 and TLR7, as two major mediators of inflammatory response, and the targets that can be used for prognosis of HCV infection and additional anti-HCV targets.
2. Objectives
The aim of this study was to determine the mRNA expression level of TLR2 and TLR7 in HCV-infected patients, who were not under therapy, according to the possible effect of treatment on the expression level of TLR2 and TLR7 in comparison with normal controls. In addition, we investigated whether there is an association between the mRNA expression level of TLR2 and TLR7 with HCV viral load and different genotypes. The obtained results could introduce an important new set of molecular markers for predicting future trends of the regulation of TLR and molecular targets for HCV vaccine and vaccine adjuvant or for the treatment of this infection.
3. Patients and Methods
3.1. Patients
This study was carried out as a case-control study on two different groups. Group І consisted of nineteen healthy controls and group П consisted of nineteen consecutive new cases of patients with HCV infection referred to Bou-Ali Pathobiology Laboratory as the reference molecular laboratory in Yazd, Iran. All samples were examined by a gastroenterologist, and lack of fibrosis was detected using the Scheuer scoring system (18). The patients had Caucasian ancestry and did not show apparent auto-immune hepatitis, alcoholic liver disease, primary biliary cirrhosis, sclerosing cholangitis, Wilson’s disease, α1-antitrypsin deficiency, decompensated cirrhosis, overt hepatic failure, current or past history of alcohol abuse, and previous liver transplantation or evidence of hepatocellular carcinoma. Five milliliters of each peripheral blood sample was taken and centrifuged after clotting and the serum was harvested and stored at -70°C until use. Sera were screened for hepatitis B surface antigen (HBsAg) using a chemiluminescence assay (LIAISON DiaSorin SpA, Italy). Anti-Human immunodeficiency virus (HIV) antibodies were detected using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Diapro, Diaplus, Italy). Positive samples for HBsAg and HIV were excluded from this study. All samples seropositive for anti-HCV antibody, confirmed using a commercial ELISA kit (Diapro, Diaplus, Italy), were selected. None of the patients had received antiviral treatment before entry into the study in order to avoid the possible effect of therapy on the expression of TLRs. Seropositive subjects for HCV antibodies were analyzed using the Real-Time Polymerase Chain Reaction (RT-PCR) method. The plasma viral load samples were determined in accordance with the manufacturer’s instruction (Aj Roboscreen GmbH, Germany). The Rotor-Gene 6000 (Corbett Research, Sydney, Australia) was used to determine the viral load level. Analytical sensitivity of the kit was 200 copies/mL. Hepatitis C Virus-RNA-positive samples were genotyped using the HCV genotypes kit (Sacace Biotechnologies S.r.l, Italy). The kit was able to determine HCV genotypes 1a, 1b, 2, 3, and 4. Serum alanine aminotransferase (ALT) levels in HCV patients were measured by the Prestige auto-analyzer, (Tokyo, Japan) and the biosystem kit (Spain). Nineteen sex and age-matched healthy subjects, sero-negative for HBsAg, HIV and HCV were enrolled as controls. The Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved this protocol. All patients signed a written informed consent for the study.
3.2. RNA Extraction and Reverse Transcription
Two milliliters of venous blood was taken from all subjects who were HCV-RNA positive and transferred to ethylenediaminetetraacetic acid (EDTA)-containing tubes. Total RNA was extracted using the gene JET RNA purification kit (Thermo Scientific, Belgium). The quantity and purity of the RNA were assessed by measuring absorbance at 260 nm and the ratio A260/A280 in a UV-spectrophotometer (PhotoBiometer, Eppendorf, Germany). A total of 1µg of RNA was reverse transcribed into single-stranded complementary DNA (cDNA) using a commercial kit (Revert Aid First Strand cDNA Synthesis kit, Thermo Scientific, Belgium). The products were analyzed on 1% agarose gel stained with a green viewer.
3.3. Real-Time Polymerase Chain Reaction
The RT-PCR was performed using the Real Q-PCR Master Mix (7 mM MgCl2) kit (Ampliqon III, Denmark), according to the kit protocol, and the mRNA expression level of TLR2 and TLR 7 were quantified using the ABI/PRISM 7000 sequence detection system (Applied Biosystems, Foster, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene for normalization of the amount of mRNA expression of the gene of interest. Primers were designed according to published sequences in the Genome Database (Genbank) using the PRIMER3 software. The oligonucleotide primers for GAPDH, TLR2 and TLR7 are shown in Table 1. Briefly, 2 μL of cDNA was used for each PCR with 250 nM of forward and reverse primers in a total volume of 20 μL. The thermal cycling conditions comprised of 10 minutes at 95°C, followed by 45 cycles at 95°C for 15 seconds, 56°C for 60 seconds, and 72°C for 15 seconds. To control for specificity of the amplification products, after 45 cycles a melting curve was generated in the range of 55°C to 95°C. Fast loss of fluorescence was observed at the denaturing/melting temperature of the DNA fragment, which is a unique feature of that fragment. The relative quantity of the target mRNA was normalized to the level of the internal control GAPDH mRNA level. Gene expression was expressed in relative units (RQ = 2-ΔΔCT) where ΔCT is the difference between the gene of the target and the housekeeping gene, and ΔΔCT is the change between the ΔCT for each sample and the control group. Each TLR2 and TLR7 gene was then described as the fold change from the control group. Analyses were performed with the Step One Software v 2.1 (Applied Biosystems).
| Gene | Primer sequence (5’ to 3’) | Length | Temperature | GC, % | Amplicon length. |
|---|---|---|---|---|---|
| TLR2 | Forward: GGGTTGAAGCACTGGACAAT | 20 | 58.4 | 50.0 | 81 |
| Reverse: TTCTTCCTTGGAGAGGCTGA | 20 | 58.4 | 50.0 | ||
| TLR7 | Forward: GGCAGACCTTGGATCTAAGTAAAA | 24 | 61.8 | 41.67 | 108 |
| Reverse: GGCTAATGAGATTTCCTGACAGAT | 24 | 61.8 | 41.67 | ||
| GAPDH | Forward: GTATGACAACGAATTTGGCTACAG | 24 | 61.8 | 41.67 | 119 |
| Reverse: GTCTCTCTCTTCCTCTTGTGCTCT | 24 | 65.3 | 50.0 |
3.4. Statistical Analysis
The results were analyzed by the SPSS v.16.0 software (SPSS Inc, Chicago, IL, USA). Continuous variables (age and ALT) are presented as mean (± SD) and viral load as mean ± Standard Error of the mean (SEM). We used paired sample t-test for comparison of cycle threshold mean between patients and the control groups. Correlations between the viral load and the mRNA expression levels of TLR2 and TLR7 were determined using the Spearman test. Mann-Whitney U test was used for determining associations between genotypes of the virus and the mRNA expression levels of TLR2 and TLR7. P values of less than 0.05 were considered statistically significant.
4. Results
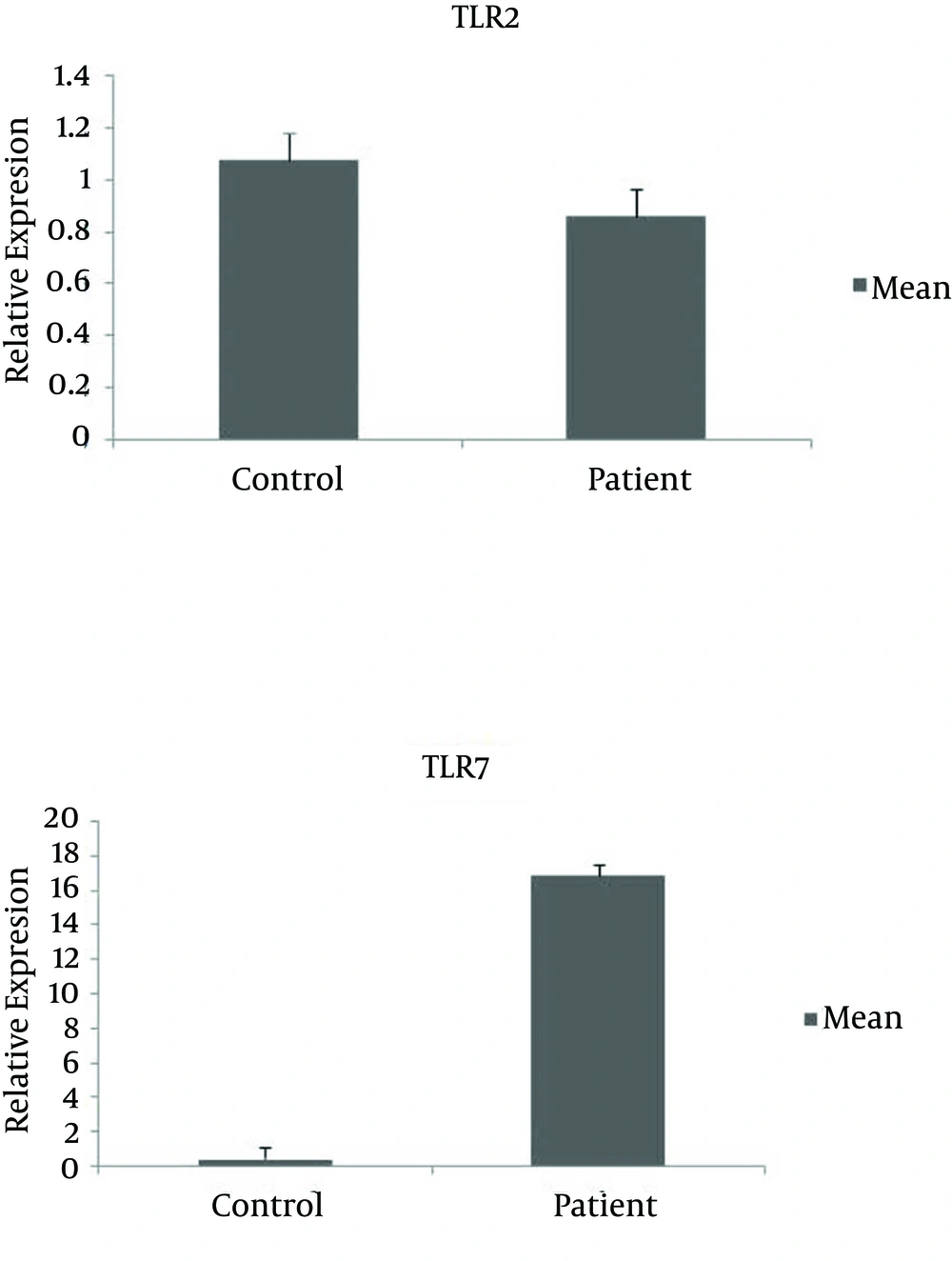
Both the HCV-infected patient group and the control group consisted of 18 males and one female. The mean age of the case and the control group was 31.8 ± 5.7 and 32.1 ± 5.8 years, respectively. The characteristics of the subjects are shown in Table 2. All samples (19 patients and 19 healthy controls) had detectable expression of TLR2 and TLR7 mRNA. The mRNA expression level of TLR7 in the case group was significantly increased, in comparison to the control group (P = 0.02), while the mRNA expression level of TLR2 was similar between the two groups (P = 0.8) (Figure 1). Evaluating the specificity of the amplification products by dissociation/melting curve did not show any amplification of unspecific products. No associations were found between mRNA expression levels of TLR2 and TLR7 and HCV viral load (P = 0.6). The mRNA expression level of TLR2 was similar in different genotypes (P = 0.9). Also, there was no significant difference between the mRNA expression level of TLR7 and different genotypes (P = 0.5). There was no significant difference in the viral load and genotypes of 1 and 3 (P = 0.3).
| Demographic Variables | Groups | |
|---|---|---|
| Group I (HCV Patient) | Group II (Control) | |
| Age, y | ||
| Mean ± SD | 31.8 ± 5.7 b | 32.1 ± 5.8 |
| Range | 21 - 43 | 24 - 43 |
| ALT, IU/L | ||
| Mean ± SD | 55.5 ± 6.1 c | 23 ± 1.9 |
| Range | 21 - 129 | 12 - 36 |
| Gender d | ||
| Male | 18 (94.7) | 18 (94.7) |
| Female | 1 (5.3) | 1 (5.3) |
| HCV Genotype d | ||
| 1 | 7 (36.8) | - |
| 3 | 12 (63.2) | - |
| Viral Load, copies/mL | ||
| Mean ± SD | 3.07 × 106 ± 1.18 × 106 | - |
| Range | 275 - 2.2 × 107 | |
aAbreviations: ALT, alanine aminotransferase; HCV, Hepatitis C virus.
bP Value = 0.8.
cP Value = 0.001.
dValues are presented as No. (%).
5. Discussion
Detection and clearance of invading pathogens rely on the cooperative interactions of innate and adaptive immunity. Toll-Like Receptors, which have broad specificity for structurally-conserved pathogen components, are involved in the interplay of these two arms of host defense (19). Various elements can influence the expression and activities of TLRs. Some of the viral proteins of HCV are able to stimulate TLR signaling, which plays an important role in viral immune clearance (8).
In this study, we found that there was a highly significant increase in TLR7 mRNA expression in peripheral blood of HCV-infected samples compared to the control group. Toll-Like Receptor 7 recognizes single stranded-RNA-viruses, including HCV and HCV genomic RNA and has direct immunostimulatory effects on TLR7 and TLR8, leading to Interferon (IFN)-α production and activation of interferon regulatory factor and NF-ϰB. The data presented in this study indicated the upregulation of TLR7, and the results were in agreement with some other studies. Dolganiuc et al. showed that RNA levels for TLR2, TLR6, TLR7, TLR8, TLR9 and TLR10 mRNA were up regulated in both monocytes and T cells in HCV-infected patients compared to controls. The TLR7 mRNA was significantly up regulated in HCV patients compared to controls (13). Also, Sato et al. reported that the expression level of TLR4, TLR7 and TLR8 in CD14+ monocytes of Peripheral Blood Mononuclear Cells (PBMCs), from HCV-infected patients, significantly increased compared to those of controls, while the expression levels of the other TLRs were similar between the patients and the controls (20). In contradiction to these results, Abdel-Raouf et al. showed that there was a highly significant decrease in TLR7 mRNA expression in PBMCs of HCV-infected subjects compared to the control group and in hepatocellular carcinoma group (21). These findings of Abdel-Raouf et al. were in agreement with the results of Atencia et al., which found a significant down regulation in TLR3 and TLR7 mRNA levels in chronic HCV infection with cirrhosis compared to healthy controls (14). Moreover, Kang et al. found that the incubation of PBMCs with HCV core proteins triggers the expression of TLR2 and suppresses TLR4 and TLR7 (22). The main reason for differences in the results of these kinds of studies was that HCV has strategies to avoid activation of antiviral pathways by TLRs and their ligands. Hepatitis C Virus selectively impairs innate immunity pathways that limit HCV replication, same as type I IFNs, while at the same time generates a chronic inflammatory state and causes persistent liver injury. In another study, Firdaus et al. analyzed the mRNA expression of TLR 3, TLR7 and TLR8 from whole blood at different stages of HCV infection including chronic, cirrhosis, and in interferon treated resolved and relapsed cases. The results showed significant up regulation of TLR7 mRNA in individuals who have progressed to the liver cirrhosis stage, compared to those whose infection had spontaneously cleared. Also, TLR7 mRNA expression level was up regulated by 2.48, 2.44, and 3.26 folds more than the control values in IFN-induced, relapsed, and cirrhosis patients, respectively (23). Thus, these findings suggested that TLR7 may be implicated in the pathogenesis of HCV infection. Recent results revealed that HCV RNA has the potential to trigger TLR7 and TLR8 in dendritic cell populations, and initiate an innate immune response against HCV infection that leads to an IFN-dependent suppression of viral replication. Taken together, the results of some previous studies suggest that excessive stimulation of TLRs reduces the expression of TLRs for several hours and could explain why cells co-stimulated with both agonists of TLR7 and TLR9 did not secrete proinflammatory cytokines during the re-stimulation. Thus, as TLR7 and TLR9 could be simultaneously or repeatedly activated, the tolerance induced through these endosomal TLRs could serve defective innate immune mechanisms in chronic viral infections (24, 25). Macrophages possess the capacity to detect HCV, in part because of surface expression of Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN). Interestingly, stimulation of naive, uninfected human macrophages with HCV core induces Tumor Necrosis Factor (TNF)-α and Interleukin (IL)-6 production through a TLR2-mediated pathway. In turn, macrophages produce inflammatory cytokines that nevertheless fail to inhibit HCV replication due to the lack of IFN-β production. Moreover, inflammatory cytokine production by macrophages upon HCV stimulation in vitro suggests that activation of macrophages may contribute to inflammation in HCV infected individuals. Therefore, these two receptors may have completely different roles in HCV infection at different stages as well as the treatment of infection (16, 26).
In the other part of this study, we determined the expression level of TLR2 mRNA in samples and found no significant difference in the expression level of TLR2 mRNA in the two groups. Some studies have demonstrated that TLR2 expression level on different cells was increased in HCV infection, and TNF-α production could promote TLR2 expression (12, 27). Wang et al. (12) showed an increase in TLR2 and TLR4 expression levels during HCV infection, and Shehata et al. (28) reported on an increase in TLR2 and TLR4 mRNA transcript level. Their results indicated that elevated expression level of TLR2 and TLR4 in chronic hepatitis C could also increase the production of inflammatory cytokines such as IFN-β, TNF-α, IL-6 and IL-8, which causes excessive inflammation and tissue injury (12, 28). An explanation for the non-significant mRNA expression of TLR2 was provided by the results of Sato et al. that showed no enhancement of TLR2 expression level in PBMC of HCV-infected patients, despite increased expressions of inflammatory cytokines such as TNF-α, IL-6 and IL-12. The results suggested that the TLR2-mediated downstream signaling pathway may be affected. In contrast, increased TLR2 expression level was found in PBMC in cirrhotic patients, whether the etiology of cirrhosis was HCV or alcohol intake. These findings may also suggest that cytokine production and TLR2 expression are augmented in patients with liver disease and enhanced shunting of gut-derived bacterial products (20), thus the studied samples did not have advanced cirrhosis. Another explanation was the effect of PEG IFNα and ribavirin therapy on the mRNA expression level of TLRs. Hammond et al. showed that PEG IFNα and ribavirin therapy could increase the mRNA expression level of TLR2, TLR4 and TLR9 for all T cell sub-populations (29). In another study by He et al. (19) it was indicated that chronic hepatitis C patients were divided to two groups of non-responders and sustained virological responders according to the virological outcome of the treatment, and the authors attempted to assess whether TLR mRNA expression level before treatment was associated with sustained virological response. They selected 15 chronic hepatitis C patients treated by a 48-week treatment with PEG IFN a-2a and ribavirin. The results demonstrated that TLRs mRNA levels are differentially expressed in baseline PBMC of chronic HCV-infected subjects with or without response to antiviral therapy (19). According to the results of these studies, we can conclude that treatment may have an effect on the expression level of TLR 2 and TLR7. The studied samples had not received any therapy and thus, the role of treatment was not considered in the present study.
We investigated the association between HCV viral load and the mRNA expression levels of TLR2 and TLR7. Our results showed no associations between mRNA expression levels of TLR2 and TLR7, and HCV viral load. Our findings confirm the results of other researchers (19, 29, 30). Also, we showed no association between the mRNA expression levels of TLR2 and TLR7, and HCV genotypes. These results were in agreement with the study Hammond et al. (29) and Berzsenyi et al. (30). Hepatitis C Virus genotype 3 was predominant in this study. There are some reports that show predominant genotypes 1 and 3 in different parts of Iran (31, 32). Hadinedoushan et al. reported that HCV genotype 3 was the predominant genotype followed by the subtypes 1a and 1b in the Yazd province (4). We did not find a significant difference be-tween mean viral load levels of patients infected with genotype 3 and those infected with genotype 1. Our results were in agreement with other reports (4, 33, 34). However, the results of the present study disagree with the findings of Chakravarti et al. They reported that the mean viral load in patients infected with HCV genotype 1 was higher than those infected with genotypes 2 and 3 (35). Although further community-based cohort studies including all genotypes of HCV virus are needed to confirm these findings. Our findings indicate that HCV infection can lead to increased expression level of TLR7 mRNA in peripheral blood cells of HCV-infected patients. We could not find a relationship between expression of TLR2 and TLR7 with HCV viral load and HCV genotypes, which means the viral load and genotypes of the virus did not affect the mRNA expression levels of TLR2 and TLR7.
More comprehensive studies will be required to demonstrate direct evidence of the relationship between the expression of cytokine and TLRs using various kinds of HCV protein and to examine the effects of various parts of the virus on TLR receptors in different cells of the immune system. Moreover, there is a need for greater focus on different cells of the immune system, as well as the evaluation of patients at different stages of the infection and therapy and in various levels of the viral load and with different genotypes of the virus.