1. Background
Hepatic encephalopathy (HE) represents a major cause of morbidity and mortality in patients suffering from chronic liver diseases. It is characterized by serious neuropsychiatric and neurocognitive complications, including confusion, disorientation, ataxia and coma (1). A considerable percentage of patients with chronic liver diseases suffered from early stage of HE, known as covert HE (2). It is characterized by poor health-related quality of life, impaired daily functioning, poor socio-economic status, susceptibility to vehicle accidents and also, increased occurrence rate of overt HE, leading to increased mortality in patients with liver cirrhosis (3-8). Therefore, early diagnosis of psychosocial impairment is crucial in cirrhotic patients to prevent further progression and potential life threatening risk.
Currently, liver biopsy is the gold standard method for the detection of liver fibrosis; however, invasiveness is an important drawback in implementing this method, particularly in patients with early stages of liver fibrosis, as well as sampling error and variability in results interpretation (9). Liver stiffness measurement (LSM) by transient elastography is an easy-to-perform, non-invasive procedure, which represents a valuable screening method for early diagnosis of fibrosis in chronic liver diseases and also, liver transplantation patients (10-13).
There is no consensus regarding a gold standard test for early diagnosis of the neuropsychiatric changes, associated with minimal liver encephalopathy. Indeed, there are several confounding factors, which strongly affect patient’s performance in neuropsychiatric tests, including age, gender, level of education and socioeconomic status. In addition, defining a cutoff point to clarify abnormal performance is difficult, due to heterogeneity of populations. Other factors, such as presence of psychiatric illnesses, other significant comorbidities and using interferon could alter the patient’s performance on cognitive tests, making interpretation of results more difficult (14, 15).
2. Objectives
The main purpose of this study was to evaluate cognitive function in patients with liver cirrhosis.
3. Patients and Methods
3.1. Study Participants
All subjects aged 25 and over, referring for liver elastography in Shariati outpatient clinic, Tehran, Iran, between March 2014 and August 2014, were invited to participate in the study. They received verbal information on the study and patients who accept to participate in this study gave their written consent. The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Patients, who accepted to participate in the study, underwent LSM and had a complete clinical evaluation and laboratory tests. The diagnosis of cirrhosis was established based on LSM findings (score >14), as well as clinical and laboratory findings. Patients presenting any of the following conditions were excluded: abdominal ascites, previous history of HE, hepatocellular carcinoma, any neurologic diseases such as Alzheimer’s disease, Parkinson’s disease and non-hepatic metabolic encephalopathies, current administration of psychoactive drugs, such as antidepressants or sedatives, alcohol and opium abuse and mental retardation. A total of 37 consecutive cirrhotic patients, with age, sex and education-matched healthy controls, were enrolled. This study was approved by the Ethics Committee of Digestive Disease Research Institute of Tehran University of Medical Sciences, Tehran, Iran.
3.2. Cognitive Function Assessment
All participants underwent Wechsler Memory Scale (WMS) test and simple visual reaction time (RT) test. The WMS is designed for assessing learning memory and working memory, which compromises of seven subsets: information, orientation, mental control, logic memory, digits forward and backward, visual reproduction and associate learning. The WMS provides a total “memory quotient” (MQ) that accounts for age-related mnemonic variability (16). Simple visual RT was determined by measuring the latency between presenting a visual stimulus and pressing a key, as the response, with the software designed on the basis of the World Health Organization-Neurobehavioral Core Test Battery (17).
3.3. Statistical Analysis
Data were analyzed using IBM SPSS Statistics software package version 20 (IBM, New York, NY, USA). One-sample Kolmogorov-Smirnov test was used to estimate the distribution type of the data. Independent sample T-test was used to investigate the probable difference of quantitative variables between groups. Chi-squared test or Fisher's exact test were performed to evaluate the difference of categorical variables between groups, as indicated. One-way analysis of variance (ANOVA) test was used to investigate the probable differences of WMS subscales among cirrhotic patients with different underlying cause.
4. Results
Of 37 subjects, 21 (57%) were male, with mean ± SD age of 46.1 ± 10.5. Demographic features for patients and controls are summarized in Table 1. The most common etiology of cirrhosis is hepatitis C virus (HCV) diagnosed in nine (25.7%) patients, followed by autoimmune hepatitis in seven (20%), hepatitis B virus (HBV) in six (16.2%) and non-alcoholic steatohepatitis (NASH) in 10 (27%). The cause of cirrhosis in five (13.5%) subjects was unknown. Twenty-six (70.3%) patients were categorized in Child-Pugh A and 10 (27%) patients in Child-Pugh B. We could not categorize one patient because of insufficient data. Two patients were excluded due to psychiatric comorbidities. There is a significant difference between two groups with respect to aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, prothrombin time, transient elastography (TE) score and controlled attenuation parameter (CAP) score (Table 1).
| Cirrhotic Patients | Non-Cirrhotic Controls | P Value | |
|---|---|---|---|
| Number, n | 37 | 37 | |
| Age, y | 49.3 ± 10.5 | 46.2 ± 9.6 | 0.1 |
| Gender | 0.8 | ||
| Male | 22 | 21 | |
| Female | 15 | 16 | |
| BMI, kg/m² | 26.6 ± 6.5 | 25.5 ± 4.9 | 0.4 |
| Level of education | |||
| Illiterate | 1 (2.7) | 1 (2.7) | |
| Primary | 5 (13.5) | 2 (5.4) | 0.1 |
| Secondary | 17 (45.9) | 11 (29.7) | |
| Tertiary | 14 (37.8) | 23 (62.2) | |
| Cirrhosis etiology | |||
| HCV | 9 (24.3) | - | - |
| HBV | 6 (16.2) | - | - |
| Autoimmune | 7 (18.9) | 1 (2.7) | - |
| NASH | 10 (27) | 28 (75.7) | - |
| Other | 5 (13.5) | 8 (21.6) | - |
| AST, IU/L, median (range) | 97.8 (20‒232) | 31.2 (8‒111) | <0.001 c |
| ALT, IU/L, median (range) | 94.9 (13‒364) | 45 (12‒120) | 0.002 c |
| ALP, U/L (range) | 368.7 (53‒2644) | 163.5 (86‒245) | 0.02 c |
| Albumin, g/dL (range) | 3.4 (1.2‒5) | 3.8 (3‒4.9) | 0.08 |
| Total Bilirubin, mg/dL (range) | 1.5 (0.5‒4.4) | 0.9 (0.4‒1.8) | 0.001 c |
| Prothrombin time, s | 13.9 (11.5‒18.1) | 12.4 (12‒14.4) | 0.001 c |
| TE scores, kPa | 33.9 ± 19 | 5.2 ± 1.2 | <0.001 c |
| CAP Score | 232.6 ± 52.8 | 271.9 ± 43.4 | 0.001 c |
The MQ rate was significantly lower in patient group compared to control (P <0.001). The result for the subsets of WMS demonstrated that information (P = 0.02), orientation (P = 0.01), mental control (P = 0.001), logical memory (< 0.001), and visual reproduction (P = 0.001) were significantly lower in cirrhotic patients, although, in case of associate learning, both groups showed similar results (Table 2). Comparing HCV cirrhotic subjects to other underlying cause of cirrhosis demonstrated that there is a significant impairment in logical memory, visual reproduction and MQ score (P = 0.01, P = 0.04, P = 0.01, respectively). However, when we compared all etiologies with each other, we did not find any statistically significant difference for all WMS subscales. Again, when we categorized etiology of cirrhosis based on viral and non-viral and put HBV and HCV in one group, comparing to other non-viral etiologies, cognitive impairment between these groups did not show any significant difference.
| WMS Subsets | Cirrhotic Patients | Healthy Controls | P Value |
|---|---|---|---|
| Information | 5.5 ± 1 | 5.9 ± 0.2 | 0.02 b |
| Orientation | 4.6 ± 0.7 | 4.9 ± 0.1 | 0.01 b |
| Mental control | 5.4 ± 2.6 | 7.3 ± 1.8 | 0.001 b |
| Logical memory | 6 ± 2.7 | 9.6 ± 3.7 | < 0.001 b |
| Digits forward and backward | 7.7 ± 3.1 | 10.4 ± 2 | < 0.001 b |
| Associate learning | 14.6 ± 15.8 | 16.4 ± 4 | 0.4 |
| Visual reproduction | 6.4 ± 4 | 9.2 ± 3.1 | 0.001 b |
| Memory quotient | 91 ± 18.2 | 114.6 ± 17.5 | < 0.001 b |
Scores of Wechsler Memory Scale Subsets for the Patient and Control Groups a
The results of simple RT were significantly better (faster) in control subjects compared to cirrhotic patients (483.4 ± 188.8 vs. 371.2 ± 79.8, P = 0.002). Furthermore, the number of errors was significantly higher in HCV patients compared to other groups (P = 0.003)
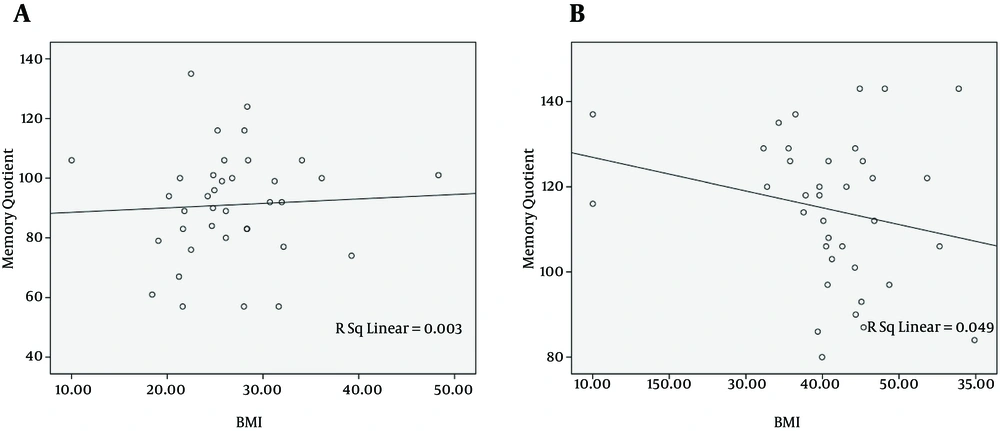
In the next step, the probable association between MQ score and BMI was investigated. There is a positive relationship between MQ score and BMI in cirrhotic patients (r = 0.054, Figure 1 A). On the other hand, this relation showed an inverse association in non-cirrhotic individuals (r = -0.222, Figure 1 B). These relationships, however, were not statistically significant (P = 0.75 and P = 0.18, respectively).
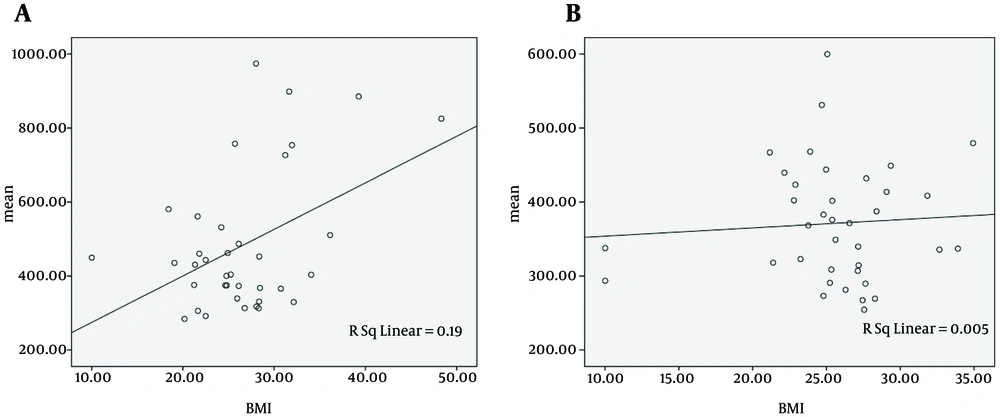
Regarding the probable association between mean RT and BMI, both cirrhotic patients and non-cirrhotic individuals with higher BMI showed slower performance. (r = 0.436, P = 0.007 and r = -0.069, P = 0.68, respectively) (Figure 2).
5. Discussion
In this study, for the first time, neurocognitive function of patients with liver cirrhosis was evaluated by WMS and simple visual RT tests. Moreover, in previous studies regarding cognitive assessment of patients with covert HE, the diagnosis of cirrhosis was confirmed by liver biopsy; however, this is the first report in which TE was used to confirm the diagnosis.
Our findings are consistent with the previous studies, with respect to cognitive function alteration in patients with covert HE (18, 19). In WMS test, cirrhotic patients received an average score of 91 ± 18.2, while the control group had an average score of 114.6 ± 17.5. Therefore, this assessment reliably differentiates subjects in early stage of HE from non-cirrhotic subjects. Similar to WMS, simple visual RT test results are significantly different in cirrhotic patients, compared to healthy individuals. This test has a clear advantage over the other methods as it evaluates the visual and motor response and it appears reasonable that the test results are less influenced by confounding factors.
When we compared cognitive function among groups with different underlying etiology of cirrhosis, no significant association was observed. This finding is consistent with previous studies, indicating that cognitive function alteration was not significantly influenced by the underlying cause of liver cirrhosis (19-21). The association between chronic HCV infection and neuropsychiatric disorders, including cognitive impairment, has been shown in previous studies (22-24). Therefore, we categorized HCV patients, as one group, and compared their WMS subscale scores with other patients. As we expected, HCV patients’ score on WMS test was significantly lower, with respect to logical memory, visual reproduction and MQ score. Drug abuse, using interferon and disease chronicity have been proposed by many authors, as responsible factors for this relationship (25, 26), while others believed that HCV directly involves the brain (27). In our study, all patients were newly diagnosed and were not current drug users and did not receive interferon therapy. This finding further supports the evidences of brain involvement in patients with HCV (27). Recently, it has been shown that HCV infection leads to impairment in the brain derived neurotrophic factor (BDNF) production (28). This factor, as the most abundant growth factor in the brain, has an important role in the regeneration and development of neuronal cells and is thought to be responsible in the pathogenesis of cognitive problems and mood disorders (29-31).
The negative relationship between MQ score and BMI, in non-cirrhotic subjects, was consistent with previous published studies (32, 33). Surprisingly, obesity has a paradoxical effect on cognitive function in our cirrhotic patients; higher BMI was associated with higher MQ score. This finding could be potentially explained by the fact that disease progression leads to weight loss, in liver cirrhosis, and patients with higher BMI are in a better clinical condition. This finding may be biased by the presence of abdominal ascites; however, most of our patients had an early stage of liver cirrhosis and did not developed ascites.
Our study has several limitations. First, our sample size is relatively small, which might affect the external validity of our results. Second,the assessment of cognitive function was applied in a non-blinded manner.
To summarize, our results demonstrated for the first time that cognitive function, in HCV patients, was more impaired compared to other underlying etiologies of cirrhosis. Therefore, neurocognitive evaluation must be especially considered in the management of patients with HCV infection. The WMS and simple RT tests are reliable methods for the early detection of HE in cirrhotic patients; however, larger studies are essential to determine cut-off values for these tests. In addition, TE could be a feasible and non-invasive procedure in order to screen general population and early diagnosis of apparently healthy patients suffering from early stages of chronic liver disease.