1. Background
Osteopontin (OPN) is a multifunctional protein, involved in pathological conditions such as inflammation, immunity, angiogenesis, fibrosis, and cancer progression in various tissues (1). OPN, also known as early T-cell activation gene-1, is a secreted phosphoprotein 1 and has been implicated in the pathogenesis of various inflammatory and fibrotic disorders. It stimulates T-cell proliferation and induces T cells and macrophages to express other T helper type 1 cytokines during inflammation (2). OPN also induces the accumulation of extracellular matrix by binding to type I collagen, fibronectin, and osteocalcin, thereby contributing to tissue fibrosis (3). OPN comprises multiple functional domains, with a high sialic acid content, an aspartate-rich domain, a calcium-binding domain, a thrombin cleavage site, many residues with consensus for phosphorylation, and an integrin-binding arginine-glycine-aspartate motif, which play a key role in several inflammatory disorders (4). High levels of circulating OPN have been demonstrated in patients with hepatitis C virus (HCV) infection (5) as well as gastric and liver cancers (6). Nonalcoholic steatohepatitis (NASH) is a leading cause of cirrhosis. Syn et al. (7) showed that NASH-related cirrhosis is associated with the Hedgehog pathway activation. The gene-encoding OPN, a profibrogenic extracellular matrix protein and cytokine, is a direct transcriptional target of the Hedgehog pathway (7).
HCV is a major cause of post-transfusion hepatitis and liver transplantation in many countries (8). Liver-related complications such as cirrhosis and hepatocellular carcinoma (HCC) are among the major complications in HCV-infected patients (9).
HCC is a highly aggressive carcinoma of the liver; it is the fifth most common cancer worldwide and the third leading cause of cancer-related death. Risk factors for HCC include infection with hepatitis B virus (HBV) or HCV, alcoholic cirrhosis, and exposure to environmental toxins such as aflatoxins (10). Chronic hepatitis C is the most common cause of chronic liver disease and cirrhosis; it is also the most common indication for liver transplantation in the United States (US), Australia, and most of Europe (11). The Egyptian Demographic Health Survey (EDHS), a cross-sectional survey including HCV biomarkers, was conducted in 2008 on a large nationally representative sample. It estimated that the HCV prevalence was 14.7% among individuals between 15 and 59 years of age (12). Accordingly, Egypt has the highest HCV prevalence in the world (13).
2. Objectives
In this study, we aimed to assess the value of plasma OPN as a biomarker for HCC and to evaluate its diagnostic value in nonalcoholic fatty liver disease (NAFLD) and its relationship with clinical and laboratory features of HCC and NAFLD.
3. Patients and Methods
3.1. Subjects
This study was carried out in the Department of Internal Medicine, Faculty of Medicine, Cairo University, Cairo, Egypt, between May 2014 and March 2015, and included 120 subjects subdivided into 5 groups: Group I comprised 25 patients with HCV without cirrhosis; Group II included 25 patients with HCV with liver cirrhosis; Group III included 25 patients with HCC on top of cirrhosis; Group IV was comprised of 25 patients with NAFLD; and Group V consisted of 20 adult healthy age- and sex-matched control subjects. Written informed consents were obtained from all the participants.
3.2. Exclusion Criteria
The exclusion criteria were comprised of HBV and HCV coinfection; any malignant diseases other than HCC; diabetes mellitus; autoimmune or inflammatory conditions; respiratory, cardiac, or renal troubles; and pregnancy.
3.3. Methods
All the participants were subjected to full history taking, abdominal ultrasonography, and laboratory evaluation comprising complete blood count, blood sugar, bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), serum albumin, urea, creatinine, international normalized ratio, hepatitis B surface antigen, hepatitis C virus antibodies, HCV-RNA by qualitative polymerase chain reaction (for Groups I, II, and III), serum α-fetoprotein (AFP), and plasma OPN levels. Five mL of fasting (6 – 8 hour) venous blood samples was collected from each subject participating in the study and divided into 2 parts: The first part, 3 mL of blood, was put in a plain tube and left to clot, and the serum was separated by centrifugation for 15 minute at 1,000 × g to determine the liver and kidney functions and AFP. The second part, 2 mL of venous blood, was collected on ethylene diamine tetraacetate (EDTA) and centrifuged for 15 minute at 1,000 × g within 30 minute of collection and stored at -20˚C to determine plasma OPN. Complete blood count was performed on a Coulter Counter T890 (Coulter Counter, Harpenden, UK). Liver function tests (serum bilirubin, ALT, AST, and albumin) were determined calorimetrically on a Hitachi 912 AutoAnalyzer (Hitachi 912, Hitachi, Japan). Prothrombin time and prothrombin concentration were performed using the standard thromboplastin method on an automated blood coagulation analyzer (Siemens AG, Erlangen, Germany). AFP was determined using the enzyme-linked immunosorbent assay (ELISA) kit supplied by Kamiya Biomedical Company (Seattle, US) (14). Plasma OPN was determined using an immunoassay (ELISA) kit supplied by Fisher Scientific UK Ltd (Bishop Meadow Road, Loughborough, UK) (15).
3.4. Statistical Analysis
Analysis of data was done using Statistical Package for the Social Sciences (SPSS), version 16, to describe the quantitative variables (mean, standard deviation [SD], and range). Description of the qualitative variables (number and percentage) was carried out using the χ2 test. The one-way analysis of variance (ANOVA) was used to compare more than 2 groups as regards the quantitative variables in parametric data. The Spearman correlation test was utilized for the nonparametric data, while the Pearson correlation test was employed for the parametric measurements. A P ≤ 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were plotted. The area under the ROC curve (AUC) was calculated, and its 95% confidence interval (CI) was calculated via 1,000 bootstrap samples. Optimal cutoffs were calculated using the maximum sum of sensitivity and specificity and the minimum distance to the top-left corner of the ROC curve.
4. Results
The present study recruited 120 subjects comprised of 85 (70.83%) males and 35 (29.17%) females. Their ages ranged from 24 to 75 years with a mean age of 49.72 ± 19.3 years (Table 1). Apropos clinical manifestations, 44.6% of our patients had jaundice, 29.23% had a history of encephalopathy, 36% had ascites, and 43% had bilateral lower limb edema. Moreover, 52% of the patients were anemic and 47% were thrombocytopenic. Additionally, 49% of the patients had elevated liver enzymes, 44% had elevated serum bilirubin (biphasic), and 48% had decreased serum albumin.
| Variables | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Age, y c | 47 ± 11 | 53 ± 7 | 49.8 ± 8 | 48 ± 10 | 48 ± 10 |
| Gender c | |||||
| Male | 19 (76) | 18 (72) | 18 (72) | 16 (64) | 14 (70) |
| Female | 6 (24) | 7 (28) | 7 (28) | 9 (36) | 6 (30) |
| BMI d, kg/m2 | 26 ± 8 | 23.6 ± 7 | 25.5 ± 11 | 39 ± 10 | 29 ± 2.8 |
| ALT, IU/L | 44.2 ± 5 | 35 ± 10 | 31.9 ± 5 | 48.5 ± 6 | 23 ± 4.9 e |
| AST, IU/L | 46 ± 21 | 41 ± 17 | 28 ± 10 | 40 ± 6 | 16.4 ± 3.5 e |
| Bilirubin, mg/dL f | 0.94 ± 0.3 | 2 ± 0.9 | 3.2 ± 0.4 | 1.16 ± 0.2 | 0.95 ± 0.1 |
| Albumin, g/dL g | 4.5 ± 2 | 3.2 ± 1.6 | 2.6 ± 0.4 | 4.1 ± 0.3 | 4 ± 0.2 |
| PT, sec g | 12.3 ± 4 | 16.7 ± 5 | 18 ± 3 | 11 ± 2.3 | 10.8 ± 2 |
| PC, % f | 90 ± 11 | 50 ± 12 | 41.2 ± 10 | 93 ± 12 | 99 ± 10 |
| INR f | 1.03 ± 0.4 | 1.8 ± 0.6 | 2.1 ± 0.2 | 1.06 ± 0.3 | 1 ± 0.3 |
| Platelets, × 109/L g | 209 ± 50 | 135 ± 50 | 120 ± 40 | 234 ± 80 | 224 ± 67 |
| HB, g/dL g | 14 ± 2 | 11 ± 2.2 | 10 ± 0.9 | 11.8 ± 1.4 | 14 ± 1.4 |
| AFP, ng/mL | 4.1 ± 1.8 | 3.9 ± 1.3 | 771 ± 165 e | 6.8 ± 4 | 8.7 ± 7 |
A comparison of the demographic data of all the patients and controls in terms of age, sex, and body mass index (BMI) revealed that the fatty liver group had a significantly higher BMI than the other groups (P < 0.001) according to the one-way ANOVA; nevertheless, there were no statistically significant differences between all the groups concerning age and sex. Regarding the results of the clinical parameters, there were statistically significant differences between all the groups vis-a-vis jaundice, encephalopathy, ascites, and lower limb edema (P < 0.001). Concerning the results of the laboratory parameters, there were statistically significant differences in ALT and AST levels between the control group and the other groups (P < 0.001). There was a statistically significant difference in serum bilirubin between the non-cirrhotic groups (Groups I, IV, and V) and the cirrhotic (Group II) and HCC (Group III) groups. Also, there were statistically significant differences in serum albumin and prothrombin time between the control and non-cirrhotic groups and the cirrhotic and HCC groups. Furthermore, there were statistically significant differences in the platelet count and the hemoglobin concentration between the cirrhotic and HCC groups and the other groups (P < 0.001) (Table 1).
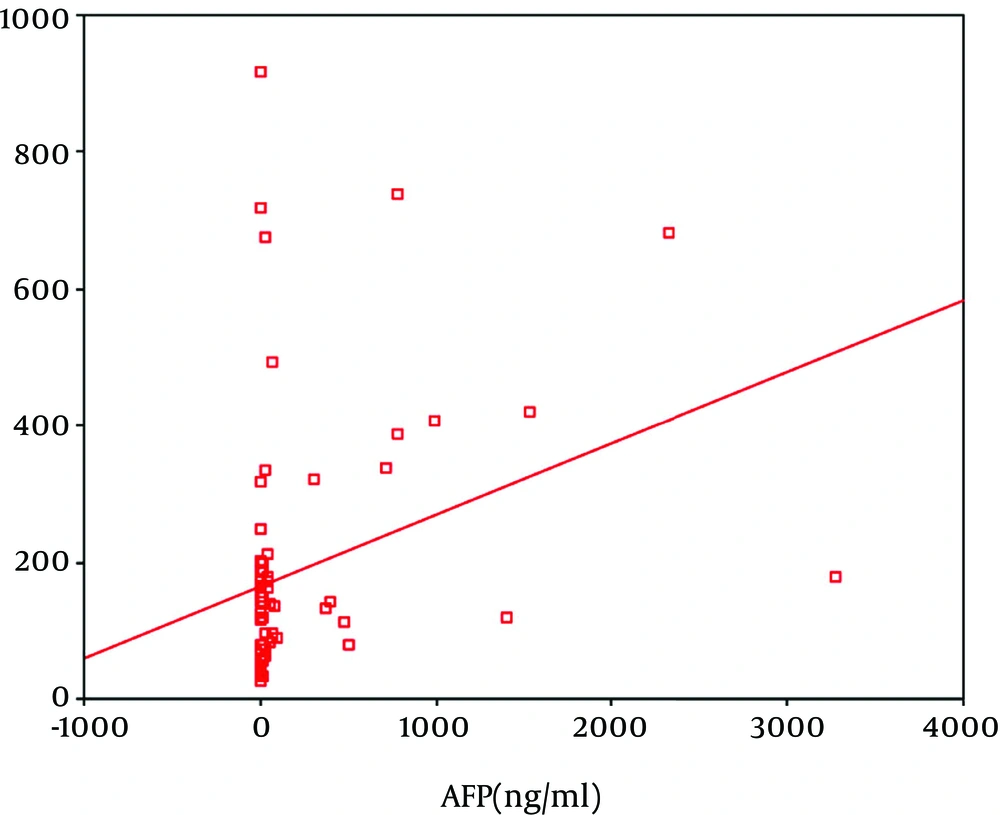
In regard to the correlation between the OSP level and the different variables in the different groups, our results revealed only significant correlations between OSP and the degree of lower limb edema in Group II (r = 0.39; P = 0.03) and between OSP and ALT (r = 0.56; P = 0.02), bilirubin (r = 0.025; P = 0.01), and AFP in Group III (r = -0.45; P = 0.03) (Table 2 and Figure 1).
| Variables | Group I | Group II | Group III | Group IV | Group V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| ALT | 0.09 | 0.12 | 0.02 | 0.56 | 0.56 | 0.02 | 0.16 | 0.26 | 0.10 | 0.20 |
| AST | 0.11 | 0.24 | 0.15 | 0.55 | 0.18 | 0.20 | 0.06 | 0.70 | 0.04 | 0.66 |
| Bilirubin | -0.03 | 0.55 | -0.07 | 0.40 | 0.25 | 0.01 | 0.15 | 0.34 | 0.19 | 0.30 |
| Albumin | 0.13 | 0.25 | -0.11 | 0.20 | -0.14 | 0.18 | 0.11 | 0.22 | 0.01 | 0.92 |
| PT | 0.14 | 0.19 | 0.17 | 0.39 | 0.12 | 0.33 | -0.08 | 0.67 | -0.02 | 0.87 |
| PC | 0.23 | 0.11 | 0.23 | 0.11 | 0.20 | 0.16 | 0.18 | 0.26 | -0.19 | 0.29 |
| INR | 0.16 | 0.33 | 0.10 | 0.30 | 0.18 | 0.19 | 0.10 | 0.22 | 0.14 | 0.25 |
| Platelets | -0.09 | 0.35 | -0.09 | 0.35 | -0.03 | 0.65 | -0.08 | 0.75 | -0.07 | 0.70 |
| AFP | -0.03 | 0.43 | -0.17 | 0.40 | 0.45 | 0.03 | 0.15 | 0.40 | 0.12 | 0.30 |
| HB | 0.14 | 0.52 | 0.18 | 0.52 | 0.14 | 0.30 | 0.10 | 0.39 | 0.15 | 0.29 |
| WBCs | 0.15 | 0.16 | 0.04 | 0.38 | 0.17 | 0.50 | 0.19 | 0.20 | 0.09 | 0.70 |
| Age | 0.21 | 0.23 | 0.02 | 0.78 | 0.19 | 0.70 | 0.07 | 0.80 | 0.03 | 0.90 |
| BMI | -0.17 | 0.40 | -0.01 | 0.80 | 0.22 | 0.15 | 0.24 | 0.11 | 0.09 | 0.81 |
| Lower limb edema | - | - | 0.39 | 0.03 | 0.06 | 0.56 | - | - | - | - |
Our results also demonstrated that AFP, bilirubin, and lower limb edema were directly correlated with the OPN level according to the stepwise technique (P < 0.05). On the other hand regarding the correlation between the AFP level and the different variables in the different groups, our results revealed only significant correlations between AFP and AST in Group II (r = 0.56; P = 0.02) and between AFP and the international normalized ratio in Group III (r = -0.43; P = 0.02).
The mean plasma OPN level in the non-cirrhotic HCV group was 168.7 ± 41 ng/mL. The mean plasma OPN level was 258.3 ± 35 ng/mL in the cirrhotic group and 401 ± 72 ng/mL in the HCC group. Additionally, the level was 106.7 ± 35 ng/mL in the fatty liver group and 106.7 ± 35 ng/mL in the control group. Our results showed that the highest level of serum OPN level was detected in the HCC group, followed by the cirrhotic group. According to the one-way ANOVA, there was a statistically significant difference in the serum OPN level between the control group and the different groups of patients. Also, a statistically significant difference in the serum OPN level was reported between the HCC group and the other groups. However, there was no significant difference in the OPN level between the patients with HCV without cirrhosis (Group I) and those with fatty liver (Group IV) (Table 3).
Mean Plasma Osteopontin Level in Studied Groups
There was a statistically significant difference in the serum OPN level between the HCC group, the benign chronic liver disease groups (Groups I, II, and IV), and the control group (P = 0.00) (Table 4).
| Plasma Osteopontin Level | Benign Chronic Liver Disease Groups (Groups I, II, & IV) (n = 75) | Hepatocellular Carcinoma Group (Group III) (n = 25) | Control Group (Group V) (n = 20) | P Values a |
|---|---|---|---|---|
| Mean ± SD, ng/mL | 177 ± 60 | 401 ± 72 | 35.1 ± 6 | 0.001 |
| Range, ng/mL | 59 - 224 | 287 - 494 | 25 - 44.9 | 0.001 |
Mean Plasma Osteopontin Level in Benign Chronic Liver Disease, Hepatocellular Carcinoma, and Control Groups
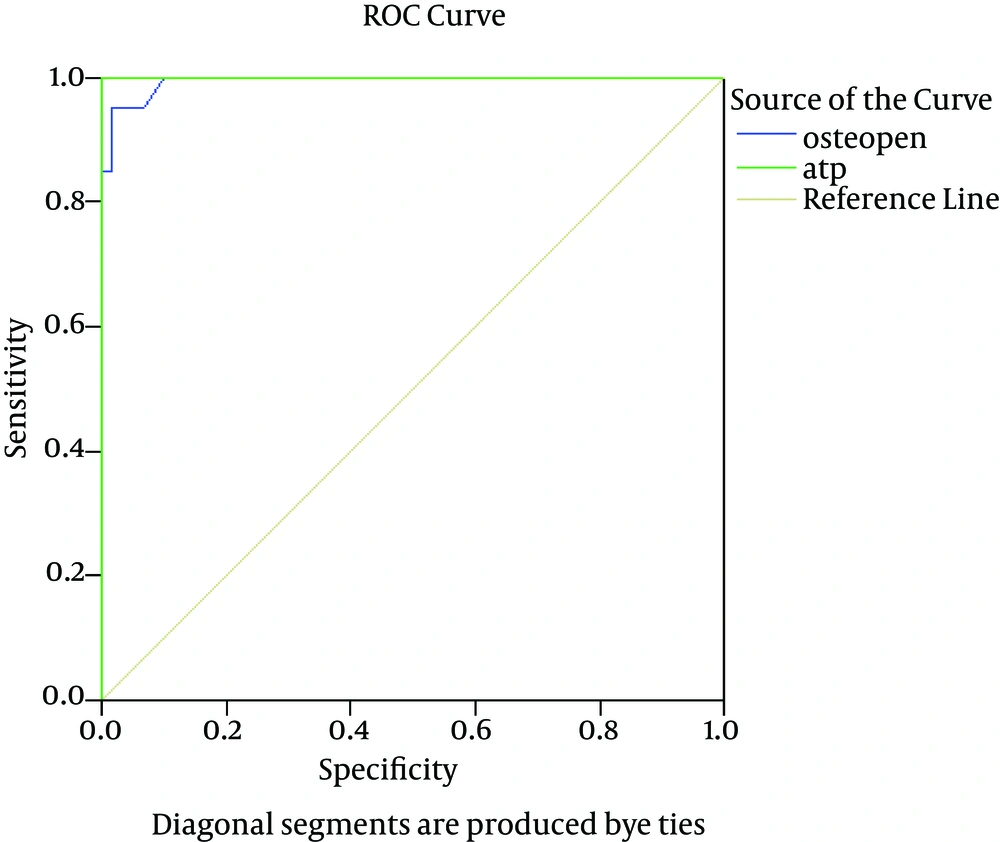
In the HCC group, the diagnostic value of OPN was comparable to that of AFP at a cutoff value of 280 ng/mL, achieving sensitivity, specificity, and overall accuracy of 100%, 98%, and 96%, respectively. In contrast, in the cirrhotic group, OPN superseded AFP by achieving 93%, 60%, and 71% for sensitivity, specificity, and overall accuracy, correspondingly (Table 5 and Figure 2).
| Variables | HCC | Cirrhosis | NAFLD | Normal | ||||
|---|---|---|---|---|---|---|---|---|
| OPN | AFP | OPN | AFP | OPN | AFP | OPN | AFP | |
| Best cutoff, ng/mL | 280 | 142 | 180 | 4 | 134 | 6.5 | 53 | 6 |
| AUC | 0.99 | 1.0 | 0.70 | 0.50 | 0.45 | 0.44 | 0.100 | 0.47 |
| Sensitivity, % | 100 | 100 | 93 | 23 | 70 | 53 | 100 | 54 |
| Specificity, % | 98 | 100 | 60 | 40 | 45 | 60 | 100 | 57 |
| PPV, % | 99 | 100 | 70 | 43 | 50 | 50 | 100 | 60 |
| NPV, % | 100 | 100 | 94 | 44 | 75 | 55 | 100 | 55 |
| Accuracy, % | 96 | 100 | 71 | 31 | 50 | 55 | 100 | 54 |
Validity of Osteopontin and Alpha Fetoprotein in the Prediction of Hepatocellular Carcinoma, Cirrhosis, and Nonalcoholic Fatty Liver Disease a
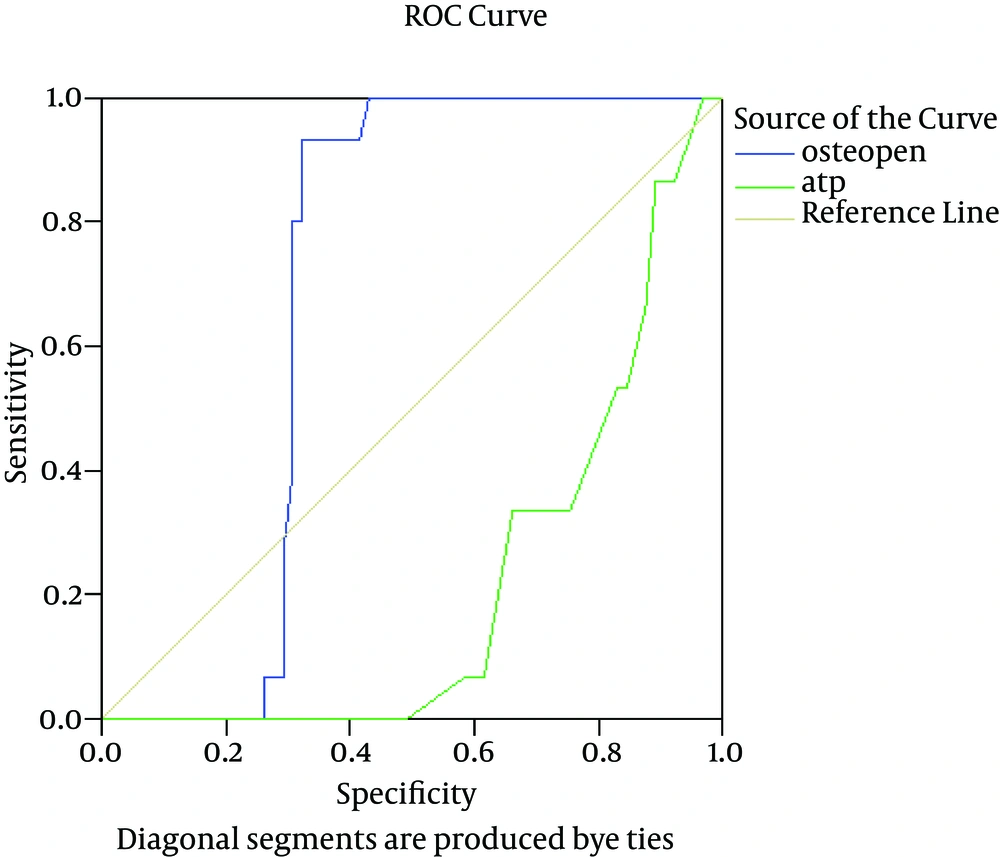
Regarding the validity of plasma OPN as a predictor of fatty change, our results revealed an AUC of 0.45% at a cutoff value of 134 ng/mL, which showed 70% sensitivity, 45% specificity, 50% positive predictive value (PPV), and 75% negative predictive value (NPV) with accuracy of 50% (Table 5 and Figure 3).
5. Discussion
The development of novel diagnostic markers for HCC has always been a significant pursuit of clinicians. The prognosis of HCC is serious with a great need for serum markers to start a therapeutic procedure at a potentially curable phase (16). Currently, AFP is the most validated serological marker for HCC, even though its performance in HCC is deficient (17). In fact, the only biochemical marker, AFP, is notoriously insufficient to detect a substantial proportion of HCC cases. Moreover, according to the practice guideline for HCC by the American Association for the Study of Liver Diseases (AASLD), AFP alone should not be used for HCC screening unless ultrasound is not available (14). Recent systematic reviews show that the quality of evidence supporting the use of AFP as a diagnostic and screening test for HCC is limited (18).
OPN, a protein encoded by phosphoprotein 1, a gene located in the same region as AFP (4q22.1 and 4q13, respectively, 14 Mb apart), was first proposed as a prognostic marker of tumor recurrence and metastasis by some investigators about a decade ago (19). Since then, it has emerged that OPN levels rise in a variety of tumor and inflammatory processes, affecting various organs and tissues (20). More recently, OPN was proposed as a novel diagnostic marker to detect liver tumorigenesis. In the liver, it has been reported that hepatic Kupffer cells secrete OPN, which facilitates macrophage infiltration into necrotic areas following carbon tetrachloride toxicity (21). OPN is an attractive potential tumor marker because it exists not only as an immobilized extracellular matrix molecule but also in a secreted form in body fluids such as plasma (22). The role of OPN in HCC has also generated a significant interest, especially with regard to its role as a prognostic factor. Moreover, recent work has highlighted the role of OPN in inflammatory liver diseases such as alcoholic and nonalcoholic disease and T-cell mediated hepatitis (23).
Our work aims to create more interest in OPN inclusion in the diagnostic process of HCC in Egyptian patients. Moreover, we sought to extend the diagnostic impact of OPN to fatty liver disease, a significant condition reported in the Egyptian population. Consequently, we tried to validate the serum OPN level in HCV patients with and without cirrhosis, HCC patients on top of chronic HCV with cirrhosis, and NAFLD patients with a view to verifying the possibility of using the serum OPN level as a potential biomarker for HCC and as a disease predictor in NAFLD.
In concordance with some other reports (24, 25), we found that the serum level of AFP was significantly higher in the HCC patients (Group III) than in the other groups, including the cirrhotic group (P < 0.05). The sensitivity and specificity of the serum AFP level in HCC detection has been shown to vary with the different cutoff values used. According to our results, at a cutoff 142 ng/mL, both the sensitivity and the specificity were 100%.
With respect to liver cirrhosis prediction, our study revealed that the sensitivity and specificity of AFP were 23% and 40%, with 43% PPV and 44% NPV. Moreover, the sensitivity and specificity of AFP in fatty change prediction were 53% and 60%, respectively, with 50% PPV and 55% NPV. We reported a significant inverse correlation between AFP and the platelet count as the patients with a low platelet count had higher levels of serum AFP. This may be explained by the progression of liver cirrhosis with a subsequent decreased platelet count (due to portal hypertension and splenomegaly).
To our knowledge, our study is one of the earliest studies to describe the predictive value of OPN in Egyptian patients with chronic liver disease and HCC. Comparing the median plasma OPN level between the different groups, we found a significant elevation in the OPN level in the HCC patients (401 ng/mL) and the cirrhotic HCV patients (258.3 ng/mL) compared to the normal control group (35.1 ng/mL). Additionally, the plasma OPN level was higher in the cirrhotic HCV patients than in the non-cirrhotic HCV group (168.7 ng/mL) and the fatty liver group (106 ng/mL). However, argument of such values was reported by other researchers (19, 22, 26-28).
Plasma OPN proved to have diagnostic accuracy in the prediction of cirrhotic liver disease. At a cutoff value of 180 ng/mL, it showed 93% sensitivity, 60% specificity, 70% PPV, and 94% NPV with overall accuracy of 71%.
NAFLD has become the most prevalent cause of liver disease in Western countries. It includes a spectrum of diseases ranging from simple steatosis to inflammatory NASH, with increasing levels of fibrosis and ultimately cirrhosis. The diagnosis of NASH can be confirmed only by liver biopsy (28). In an attempt to draw attention to the possible role of plasma OPN as a predictor of fatty change in the liver, we reported that the plasma OPN level was higher in the patients with NAFLD than in the controls (P < 0.05). At a cutoff value of 134 ng/mL, it showed sensitivity of 70%, specificity of 45%, PPV of 50%, and NPV of 75% with overall accuracy of 50%. Our results proved that OPN is sensitive in the detection of fatty change in the liver; it could, therefore, be used as a simple, noninvasive, low-cost method for the screening and early identification of NAFLD.
Regarding the diagnostic value of OPN in HCC, pioneering surveys conducted by Egyptian investigators (29-31) reported that plasma OPN could be considered a potential diagnostic biomarker for HCC in Egyptian patients with HCV. However, the authors reported different cutoff values of OPN levels (ranging from 9.3 to 300 ng/mL), which may be explained by the differences in the sample size, the study population, and the kit used to detect OPN levels. Further studies are necessary to fully explain such discrepancies.
In our study, the sensitivity, specificity, PPV, and NPV of OPN for the selective detection of the HCC group over the benign chronic liver disease groups were 100%, 98%, 99%, and 100%, correspondingly, at a cutoff level of 280 ng/mL with overall accuracy of 96%.
Our results confirm those by some previous studies (27) reporting that the diagnostic efficacy of OPN is comparable to that of AFP with a significant positive correlation between OPN and AFP levels in terms of sensitivity, specificity, PPV, and NPV. Nonetheless, some studies have found that the correlation between plasma OPN and serum AFP levels is insignificant (22, 31).
The sensitivity and specificity of OPN in HCC have been shown to vary with different cutoff values. Moreover, the reported normal median plasma OPN levels are highly variable, ranging from 31 ng/mL to even greater than 200 ng/mL, as reported in the literature (19, 22, 32). However, Matsui et al suggested that 200 ng/mL could be set as the critical cutoff point for predicting patients with HCC (20). The exact reason for these differences is not clear, but these discrepancies may be in consequence of the different assay systems and conditions of sample collection used in different studies.
Collectively, our results indicated that plasma OPN could be used in the selective detection and diagnosis of HCC. Also, OPN was superior to AFP in predicting liver cirrhosis and fatty change, which are risk factors for HCC.
In conclusion, the results of the current study revealed that the plasma OPN level was elevated in the HCV-related HCC patients by comparison to the benign cirrhotic and non-cirrhotic HCV and NAFLD patients. Notably, our data demonstrated that the plasma OPN level was elevated in NAFLD, which could be related to fatty liver change by reflecting OPN overexpression in the hepatic parenchyma. Thus, OPN represents a potential biomarker for the detection of NAFLD. Also, OPN is relatively comparable to AFP in the detection of HCC among high-risk groups and is superior to AFP in the overall prediction of chronic liver disease. Although AFP has been considered the golden standard serum marker for HCC for years, in light of our data, the usefulness of AFP testing as the only biomarker for the population at risk should be questioned.