1. Background
Hepatitis B virus (HBV), hepatitis D virus (HDV), and human immunodeficiency virus (HIV) are transmitted by blood transfusion; this is mostly due to the window period of these viruses, which makes them undetectable through the common laboratory tests (1). Hemodialysis (HD) patients are more prone to becoming the carriers of these infections because of their experience of a more nosocomial environment, sharing of dialysis machines, kidney transplantation, and frequent blood transfusions (2). In fact, any problem in disinfection or handling of appliances and inappropriate hand hygiene can lead to cross-contamination of medications and instruments. Thus, infections with the mentioned viruses are the major causes of further threats to patient safety (3, 4). Some investigations examined the prevalence of HBV, HDV, and HIV in HD patients; however, the reported values varied in different populations (5, 6). The prevalence of HBV infection in HD patients ranged from 0% to 58% in previous reports, and the value of HDV varied in convergence with HBV in most studies, since HDV requires the surface membrane proteins of HBV (7, 8). However, only a few studies have been conducted to evaluate the prevalence of HIV infection (9, 10). A report from India has shown an HIV prevalence of 1.7% in HD patients (10).
From the results of most surveys, the incidence of HBV infection in HD patients has decreased in recent years following a gentle slope. Prescription of the HBV vaccine for high-risk groups is known as a main reason of this reduction (10). Infected individuals are usually asymptomatic, with a mild clinical course. However, the severity of symptoms may differ based on the types, genotypes, and locality of these viruses (2). In this regard, hepatitis D is classified into seven major clades (HDV-1 to HDV-7) and hepatitis B virus has been categorized into eight genotypes (A - H) according to the sequence variation of the genome (11, 12). In contrast, HIV has been classified into types, groups, and subtypes by considering the genetic similarities. The two types of HIV are HIV-1 and HIV-2. Within HIV-1, four distinct groups have been identified (M, N, O, and P). Group M can also be divided into at least nine subtypes, namely A, B, C, D, F, G, H, J, and K (13, 14).
2. Objectives
Considering the importance of population and time in determining the prevalence rates of the mentioned infectious agents, the overall aim of this prospective study was to assess the prevalence of HBV and HIV infections among HD patients in Bandar Abbas, Iran, in 2015.
3. Patients and Methods
3.1. Samples and Procedures
In 2015, a total of 153 patients with chronic renal failure undergoing HD at Shahid Mohammadi hospital in Bandar Abbas were selected. Ninety-four (61.4%) patients were male and 59 (38.6%) were female. All patients provided written informed consent before enrolment in the project, and those who did not consent to participate were excluded. A complete medical history was taken from patients regarding age, sex, duration of HD, history of addiction, vaccination, blood transfusion, and using the shared HD devices. All serum samples were initially tested for HBsAg, anti-HCV, and anti-HIV over a period of 2 months.
3.2. Serologic Assays
The blood samples were investigated for aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Then, serological markers of HBV (HBsAg: hepatitis B surface antigen, HBcAb: hepatitis B core antibody, and HBsAb: hepatitis B surface antibody) and HDV infection were checked in the present research via direct immunoenzymatic assay (ELISA) of the sandwich type using commercial kits (Diapro, Italy), and samples found to be positive for HBcAb were tested for anti-HDV antibody using immunoglobulin G (IgG) antibodies, using the mentioned enzyme-linked immunosorbent assay (ELISA) kit and the competitive ELISA method. Thereafter, a fourth generation HIV ELISA based on the sandwich principle was employed to detect HIV-1 gp120, gp41, and p24 antigens, as well as HIV-2 gp36 peptide. All of the results of the specimens were confirmed by molecular tests based on polymerase chain reaction (PCR).
3.3. DNA Extraction
Viral HBV DNA and HDV and HIV RNA extraction was conducted using a Roche high pure viral nucleic acid kit (Roche Molecular Biochemicals, Germany). The extraction was performed according to the manufacturer’s instructions.
3.4. HBV-DNA Detection and HBV Genotyping
All of the specimens were examined for HBV-DNA using the PCR (core fragment) method; detection of HBV DNA was conducted using the primers described in previous reports, as follows: 2269: 5'-GGAGTGTGGATTCGCACT-3' as the sense primer (forward) and 2415: 5'-TGAGATCTTCTGCGACGC-3' as the anti-sense primer (reverse) (15). The DNA sequences of HBV were also used to determine the proper genotype according to the restriction fragment length polymorphism (RFLP) generated by the action of MboI and AvaII restriction enzymes on an amplified fragment of the PRE-S region based on Lindh et al. (16) PCR was assigned to amplify the segment from nucleotide 80 to 2823 (479 nucleotides containing the PRE-S region) by primers GenoP1 (sense, 2823 - 2845 nt, 5'-TCACCATATTCTTGGGAACAAGA-3') and GenoP2 (antisense, 80 - 61 nt, 5' TTCCTGAACTGGAGCCACCA-3'). Following this, 5 μL of the extracted DNA was added to an mixture of 1 μL of 10 mmol/L deoxyribonucleotide triphosphates, 2 μL of 50 mmol/L Mgcl2, 5 μL of 10 × Taq polymerase buffer, 1 μL (5 U) of Taq polymerase (Promega, China), 10 pmoL of each of the GenoP1 and GenoP2 primers, and 10 μL of DNA template (total volume of 50 μL). The PCR procedure started with an initial 5 minutes denaturation at 94°C, followed by 35 cycles of amplification including denaturation for 45 seconds at 94°C, annealing for 45 seconds at 58°C, and extension for 45 seconds at 72°C. Strand synthesis was completed at 72°C for 1 minute. The PCR products were incubated with the restriction enzymes MboI and Ava II (New England Biolabs, Inc., USA) for 16 hours at 37°C in a 15 μL reaction sample based on the manufacturer’s recommendations. Product amplification was performed on 3% agarose gel stained with ethidium bromide, and the products were visualized under ultraviolet (UV) and compared with Lindh patterns to determine HBV genotypes (16).
3.5. HDV-RNA Detection and HDV Genotyping
The reverse transcriptase PCR technique was carried out on serum samples to detect HDV-RNA according to a previously described strategy. Thereafter, the amplified DNA segment was purified and sequenced via a sequencing procedure, as mentioned in (17). Then, the detected nucleotide sequences were compared with the GenBank sequences to determine the genotypes of HDV, which were categorized into HDV genotypes 1 to 7.
3.6. HIV-RNA Detection and Sequencing
All of the steps in HIV-RNA detection and sequencing were performed as described by Steegen et al. The method used two forward primers (5'-GAGGAAGCTGCAGAATGGG-3' and 5'-ATGATGCAGAGAGGCAATTT-3') and two reverse primers (5'-TTCTGTATGTCATTGACAGTCCAGC-3' and 5'-TAAYTTYTGTATRTCATTGAC-3'). Thereafter, the amplification cycles were carried out and the amplified products were purified and sequenced according to the previous report (18).
3.7. Statistical Analysis
All statistical analyses were conducted using SPSS version 12 for Windows. The data are reported as means, standard deviations, and percentages. The t-test and chi-square (χ2) test were performed to analyze quantitative and qualitative variables, respectively. A two sided α = 0.05 was considered statistically significant in this investigation.
4. Results
4.1. Epidemiologic and Viral Characteristics
A total of 153 HD patients were participated in this study. Fifty-nine (38.56%) of subjects were female and 94 (61.44%) were male; the mean age of patients was 54.06 ± 15.68 years (minimum age 8, maximum age 89). Based on serologic assays, it was determined that nine (5.88%) patients were HBV positive (HBsAg-positive), whereas no HIV-or HDV-positive patients were found. HBcAb and HBsAb examinations were also positive in 99 (64%) and 54 (36%) of patients, respectively. The prevalence rates of elevated AST and ALT were 12.4% (19 patients) and 3.9% (6 patients), respectively, among the investigated HD patients. Complete demographic characteristics of the participants are given in Table 1.
| Variables | HBV Positive | HBV Negative | P Value |
|---|---|---|---|
| Age, y | 66.44 ± 10.43 | 53.28 ± 16.16 | ≤ 0.019 |
| Duration of HD, mo | 12.78 ± 13.07 | 11.90 ± 13.27 | ≤ 0.049 |
| AST , IU/L | 17.89 ± 11.25 | 19.39 ± 17.69 | > 0.05 |
| ALT , IU/L | 70.67 ± 36.72 | 45.45 ± 50.83 | > 0.05 |
| Gender | > 0.05 | ||
| Male | 6 (3.92) | 88 (57.52) | |
| Female | 3 (1.96) | 56 (36.60) | |
| Vaccination | > 0.05 | ||
| Yes | 4 (2.61) | 87 (56.86) | |
| No | 5 (3.27) | 57 (37.26) | |
| Shared HD Devices | ≤ 0.003 | ||
| Yes | 5 (3.27) | 18 (11.77) | |
| No | 4 (2.61) | 126 (82.35) | |
| Addiction | > 0.05 | ||
| Yes | 0 | 10 (6.54) | |
| No | 9 (5.88) | 134 (87.58) | |
| Blood Transfusion | ≤ 0.042 | ||
| Yes | 8 (5.23) | 121 (79.09) | |
| No | 1 (0.65) | 23 (15.03) |
aValues are expressed as mean ± SD or No. (%).
4.2. Findings of Molecular Assays for Virus Detection
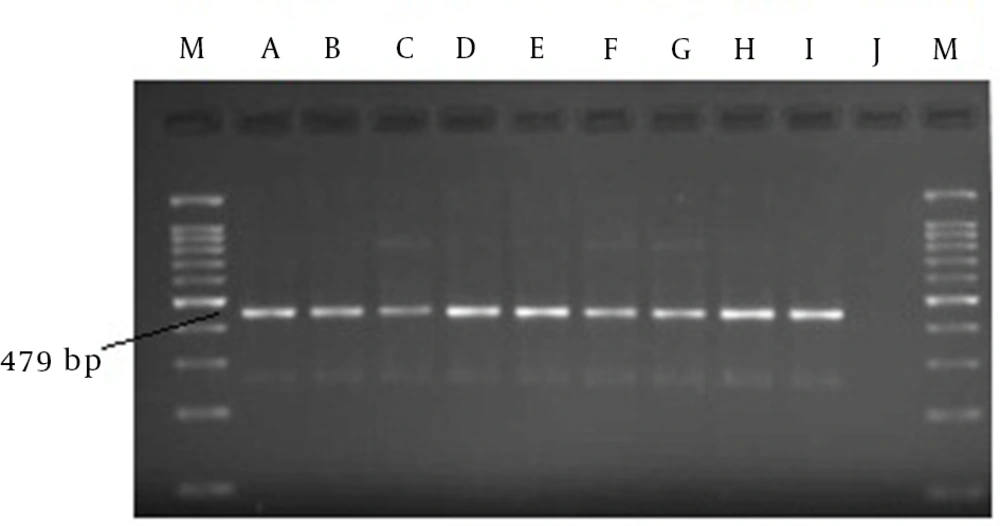
All samples were checked using molecular tests to identify positive serums. As a result, only a fragment of 479 bp was observed by electrophoresis in nine samples. These detectable segments belonged to HBV DNA. Therefore, the PCR analysis confirmed that the prevalence of HBV infection was 5.88% in this population, and none of the other viral infections were found in our examinations. Indeed, similar findings were reported for both the serologic and molecular techniques (Figure 1).
4.3. Determination of HBV Genotypes
Two RFLP patterns were diagnosed and compared with the Lindh patterns (16). Both of the patterns in present study exhibited no excision site for AvaII and two sites for MboI. The first pattern comprised three fragments measuring 123 nt, 88 nt, and 52 nt in the length. However, the second pattern consists of three segments measuring 306 nt, 88 nt, 52 nt. Based on the comparison, both of the identified patterns belonged to genotype D of HBV.
4.4. Association Between the Prevalence of the Hepatitis B Virus and the Study Variables
As shown in Table 1, further analysis of the study variables indicated a statistically significant correlation between the prevalence of HBV and age, duration of HD, history of blood transfusion, and using shared HD devices. Indeed, the mean age of HBsAg-positive patients was significantly higher than that of HBsAg-negative cases. In addition, higher duration of HD, using shared HD devices, and the experience of blood transfusion increased the risk of infection disease. However, none of the other variables illustrated a significant association in this investigation.
5. Discussion
The findings of this research in the Hormozgan province indicated that none of the patients were infected with HIV or HDV. This is compatible with recent studies in Iran (19, 20). In a project by Alavian et al. the prevalence of HBV was reduced from 3.8% in 1999 to 2.6% in 2006 among HD patients in Iran (21). In addition, the prevalence of HBV infection in the present investigation (5.88%) was close to that of HBsAg (6.72%) determined by Mostaghni et al. in Bushehr province, southern Iran (22). It is also relatively low compared with most similar studies in Asia-Pacific (23) and African countries (24, 25). However, another survey in Khartoum, Sudan demonstrated a relatively similar value (4.5%) in this regard (26). Two studies in Brazil also indicated a higher prevalence of HBV infection in HD patients (27, 28). In contrast, the observed prevalence of HBV infection in HD patients was higher than that recently reported surveys in HD patients in developed countries, including the United States (2.4%), Japan (2.2%), and most of the countries in Europe (4.1%) (29). In conclusion, projects from less developed countries showed higher prevalence rates of HBV in HD patients. Generally, the prevalence of HBV in the general population may determine the incidence and prevalence of HBV in HD patients. The prevalence in each society represents the level of success of healthcare strategies and prevention systems in that population and HD units (30).
Similar to the previous research in Khuzestan province, the distribution of HBV genotypes among HD patients was analyzed by the RFLP method in present investigation (31). In accordance with all of the previous investigations in Iran (32, 33), we could only detect genotype D of HBV. This low diversity may be due to the short evolutionary period of this genotype (34). Therefore, genotype D is the major genotype of HBV in Iran. This finding is also in accordance with those of several large epidemiological studies that determined a high prevalence of genotype D in the Middle East (35-37).
Based on the analysis, HBV-positive patients were significantly older in terms of mean age than HBV-negative ones. This result is in agreement with the most of the research on this issue (22, 38). This association may be due to the weaker immune system in older individuals. However, another investigation in Libya reported that seropositive patients were younger than seronegative ones (29).
In accordance with the findings of many authors (39), the prevalence of HBV infection in HD patients was also strongly related to a positive history of blood transfusions. In contrast, El-Ottol et al. reported that there is no association between a history of blood transfusion and HBV. In the other words, they thought that the screening of blood and blood products for HBV antigens by adequate tests reduces the incidence of HBV infection (40). The findings emphasize the importance of appropriate screening of blood donors. In addition, we can conclude that improvements in management of anemia by other treatment methods, including erythropoietin and iron therapy, may decrease the risk of infection in patients (29).
The length of time on HD and using shared HD devices were strongly associated with the prevalence of HBV seropositivity. In this regard, Mahdavimazdeh et al. reported that duration of HD treatment seems to be a significant variable in managing HBV infection in medical centers with HD facilities. This variable has been reported to be strictly correlated with the seroprevalence of hepatitis B, demonstrating the significant risk of HBV nosocomial transmission (41, 42). In addition, Sartor et al. demonstrated that using shared HD machines increases the risk of viral infection. These correlations reflected the nosocomial transmission of HBV infection and the importance of prevention systems to reduce this type of transmission (43). Further analysis of variables in the present research documented that HBV vaccinations for HD patients cannot reduce the risk of infection.
Various strategies have been developed to improve the success of HBV vaccination, including doubling the vaccine dose and co-administering zinc, gamma-interferon, thymopentin, interleukin-2, and levamisole as adjuvants. However, hepatitis B remains a major concern in HD centers. The age of vaccination is an important factor in relation to this issue (44). In line with our findings, similar studies in this issue indicated that HBV vaccination have low efficiency in old ages. Given the age of the patients in the present study, this result is coincidence with previous researches (45). In conclusion, the prevalence of HBV infection was low in the south of Iran, and genotype D was the major genotype of HBV in this country. Among the variables, age, duration of HD, history of blood transfusion, and using shared HD devices also affected the prevalence of HBV among HD patients.
This study has several limitations. For instance, the RFLP-PCR technique used for HBV genotyping was less sensitive in detecting mixtures than more up-to-date methods, such as line probe assay and direct sequencing (46).Another limitation was the small size of the study population, which was mainly responsible for the prevalence of 0% in HIV and HDV infections. Therefore, it is suggested that the sequencing method should be used to determine the HBV genotypes and choose a larger study population for further studies.