1. Background
Infection with hepatitis B virus (HBV) is a common cause of chronic hepatitis (1, 2). Because the HBV is not usually cytopathogenic by itself, chronic HBV infection (CHB) is a dynamic state of interaction between the virus and the host’s immune system. The natural course of CHB is generally divided into an immune tolerant phase, an immune clearance phase, and a residual or inactive phase. Patients with the HBV in the immune tolerant phase are asymptomatic carriers (AC), presenting persistent immune tolerance against HBV components (1). As the immune tolerant phase changes to the immune clearance phase, the patients suffer from various immunological responses to viral components, but these immunological responses do not clear the HBV because of covalently closed circular DNA preservation. Persistent HBV reproduction and inflammatory reactions in the liver result in progressive liver disease. Patients with progressive liver diseases present a spectrum of diseases, including active chronic hepatitis (ACH), cirrhosis (Cir), and hepatocellular carcinoma (HCC). The researchers have previously reported the effects of costimulation profiles from the livers of the AC, ACH, Cir, and HCC patients and demonstrated that costimulation influences immune responses in the liver and plays an important role in immune tolerance of AC patients, in immune injury in ACH patients, and in immune abnormalism in Cir and HCC patients. A prominent feature of the previous study was that a human liver with the entire disease spectrum of CHB, instead of a single AC, ACH, Cir, or HCC diagnosis, was used to investigate immunopathophysiology (3).
In addition to costimulation, toll-like receptors (TLRs) participate in HBV clearance in CHB patients. TLRs are a group of highly conserved molecules that play a critical role in the recognition of pathogen-associated molecular patterns (PAMPs) and in the activation of innate immune responses to infectious agents (4). TLR ligands are natural macromolecular components derived from pathogens and may be composed of lipids, lipopeptides, proteins, and nucleic acids. A subgroup within the TLR family, which includes TLR3, TLR7, TLR8, and TLR9, is localized in endosomes and recognizes nucleic acids, such as viral DNA or RNA (5). TLR3 signaling in non-parenchymal cells (NPCs) in the liver interacts with HBV components, which induce production of type I IFNs, including IFN-β and cytokines. IFN-β has been identified as a major antiviral factor produced by NPCs in response to TLR3 signaling and may contribute to HBV clearance in a unique way (6). Contrarily, HBV components, including HBsAg, HBeAg, HBx protein, HBV polymerase and viral particles, are able to block TLR3 signaling and to counteract IFN-β responses through positive or negative feedback loops (4, 7). However, it is not known whether TLR3 and costimulation proteins are interrelated.
Researchers have investigated CHB immune responses in the TLR3 signal pathway through the peripheral blood mononuclear cells (PBMCs) of CHB patients, through cell lines, or through animal experiments (6-13). The resulting models for HBV infection did not reproduce the entire disease spectrum in humans and did not include AC, ACH, Cir, or HCC. CHB immunopathophysiology must be demonstrated in a human liver containing the entire disease spectrum. Therefore, researchers should focus on TLR3 expression in the tissue of CHB patients, such as in PBMCs and the liver. In previous papers, different immune statuses between the liver and peripheral blood were defined as immune compartmentalization (14-17). TLR3 expression in patients’ livers, instead of peripheral blood, exhibit the actual immune status of CHB. Therefore, it is necessary to directly detect TLR3 expression in the livers of HD, AC, Cir, and HCC patients.
2. Objectives
This study investigated TLR3 expression in the livers of patients with the entire disease spectrum of CHB. Then, the interrelationship in immunopathophysiology between TLR3 and costimulation proteins was assessed.
3. Patients and Methods
3.1. Liver Samples and Patients
Patients with AC, ACH, Cir, and HCC and normal control subjects were recruited from four hospitals in Jiangsu province between January 7, 2002 and June 5, 2006 (3). AC, ACH, Cir, and HCC were defined according to the criteria reported by Lok et al. (18). Liver tissue was obtained during liver transplantations, surgeries, and biopsies. Detailed descriptions of the studied populations and the design and methods of the experiment were reported in the researchers’ previous paper (3).
3.2. Antibodies and Reagents
Antibodies for TLR3 (L13, SC - 16238) and all costimulatory proteins were purchased from SANTA CRUS Biotechnology Inc. (Santa Cruz, CA, USA) or R&D Inc. (Lorton, VA, USA). A pre-stained protein marker (P7708S) was obtained from BioLabs Co. (NE, USA), and ABC immunochemical kits were obtained from Vector Laboratories Inc. (Burlingame, CA, USA). All other reagents were bought from Sigma-Aldrich Inc. (St. Louis, MO, USA) (3).
3.3. Western Blot Analysis of TLR3 and Costimulatory Proteins in the Liver
The relative quantity of TLR3 and costimulatory proteins in the liver was detected by western blot analysis, as described by Zhong et al. (3, 19). The TLR3 working parameters for western blotting are shown in Table 1 and all working parameters of costimulatory proteins, including CD80, CD86, CD83, CD28, CTLA-4, CD40, and ICAM-1, have been previously reported by the researchers (3). The relative quantity of protein was normalized to the protein quantity for each sample using β-actin protein as an internal standard.
| Item | Primary Antibody Dilution Rate | Second Antibody Dilution Rate | Duration of Autoradiography, min |
|---|---|---|---|
| TLR3 | 1:100 (goat ant-human CD86 antibody) | 1:1500 (mouse ant-goat IG antibody) | 360 |
| β-Actin | 1:5000 (mouse ant-human β-actin antibody) | 1:15000 (goat ant-mouse IG antibody) | 10 |
Working Parameters for Western Blot Analysisa
3.4. Immunohistochemistry and Histological Representations
The liver histological sections from hematoxylin eosin-azure (HE) stains and immunohistochemistry specifically targeting TLR3 and costimulatory proteins were observed through light microscopy. The distribution of TLR3 in the liver was revealed, and lesions from necroinflammation and fibrosis were graded by the Ishak modified HAI system. In this system, the maximum possible score for necroinflammation is 18 and is 6 for fibrosis (20).
3.5. Statistical Analysis
Data were given as means (minimal - maximal). Clinical and immunological parameters were compared using a Kruskal-Wallis test, Nemenye test, and M test (Friedman). Correlations between TLR3 and costimulation proteins were assessed using the Spearman rank correlation coefficient. P Values below 0.05 were considered significant.
3.6. Ethics
This research was carried out in accordance with the declaration of Helsinki (2000) from the world medical association. The investigation was approved by participating hospital ethics committees, the health office of Nanjing municipal government, and the health office of Jiangsu provincial government. All patients or the immediate family members of liver donors signed informed consent documentation.
4. Results
4.1. The Clinic Characteristics of the Patients
Through histological observations, the AC and HD subjects presented high viral loads positive for HBeAg and negative for hepatitis in the liver or spotty lytic necrosis in the lobule (scores of necroinflammation and fibrosis: N 0 - 1 and F 0 - 1), with normal alanine aminotransferase (ALT) levels. The ACH patients exhibited increased ALT levels and severe necroinflammation and slight fibrosis (N 12 - 18 and F 1 - 2). The Cir and HCC patients showed abnormal or normal ALT levels, although the Cir patients displayed definite fibrosis and mild necroinflammation (N5 - 9 and F6), and the HCC patients presented slight necroinflammation and fibrosis (N 2 - 5 and F 0 - 2) in non-tumor tissue.
A correlation analysis indicated that the scores for necroinflammation in the patients’ livers were positively correlated with ALT levels and total bilirubin (TB) but negatively correlated with HBsAg and the HBV-DNA load in all ACH patients, implying that increased ALT levels and TB in peripheral blood and the necroinflammation severity in the liver were closely interconnected. The demographic information for all five groups is provided in Table 2. Detailed data were presented in the researchers’ previous paper (3).
| Group | HD | AC | ACH | Cir | HCC |
|---|---|---|---|---|---|
| Age (y) Means (minimal - maximal) | 37 (27 - 55) | 31 (23 - 51) | 40 (20 - 50) | 41 (28 - 56) | 49 (37 - 67) |
| F/M | 1/5 | 1/5 | 0/6 | 0/6 | 1/5 |
| HBsAg (0 - 1.0 S/CO) | Neg | 200.74 ± 141.17 | 75.14 ± 76.12 | 98.50 ± 44.08 | 112.01 ± 60.35 |
| HBeAg (0 - 0.28 PEIU/ml) | Neg | 1433 ± 1483.40 | 186.88 ± 218.20 | 76.88 ± 152.49 | 11.99 ± 16.66 |
| HBV-DNA < 2.70 copies/ml (log) | Neg | 7.88 ± 1.32 | 5.00 ± 20.4 | 5.34 ± 1.07 | 5.81±1.25 |
| TB (5.1 - 19 µmol/L) | Nor | 16.47 ± 3.73 | 107.80 ± 88.93 | 56.97 ± 29.05 | 17.77 ± 7.98 |
| DB (1.7 - 6.8 µmol/L) | Nor | 6.15 ± 1.11 | 44.25 ± 37.81 | 24.35 ± 15.38 | 6.97 ± 1.97 |
| ALT (5 - 40 u/L) | Nor | 32.53 ± 5.93 | 793.03 ± 1452.55 | 86.32 ± 59.52 | 55.78 ± 24.66 |
| Necroinflammation score(N 0 - 18) | 0.5 ± 0.55 | 0.67 ± 0.47 | 14.83 ± 2.85 | 7.67 ± 1.25 | 3.5 ± 1.12 |
| Fibrosis score (F 0 - 6) | 0 | 0 | 1.5 ± 0.5 | 6 ± 0 | 1.17 ± 0.68 |
Demography of the Patientsa
4.2. Expression of TLR3 in the Liver
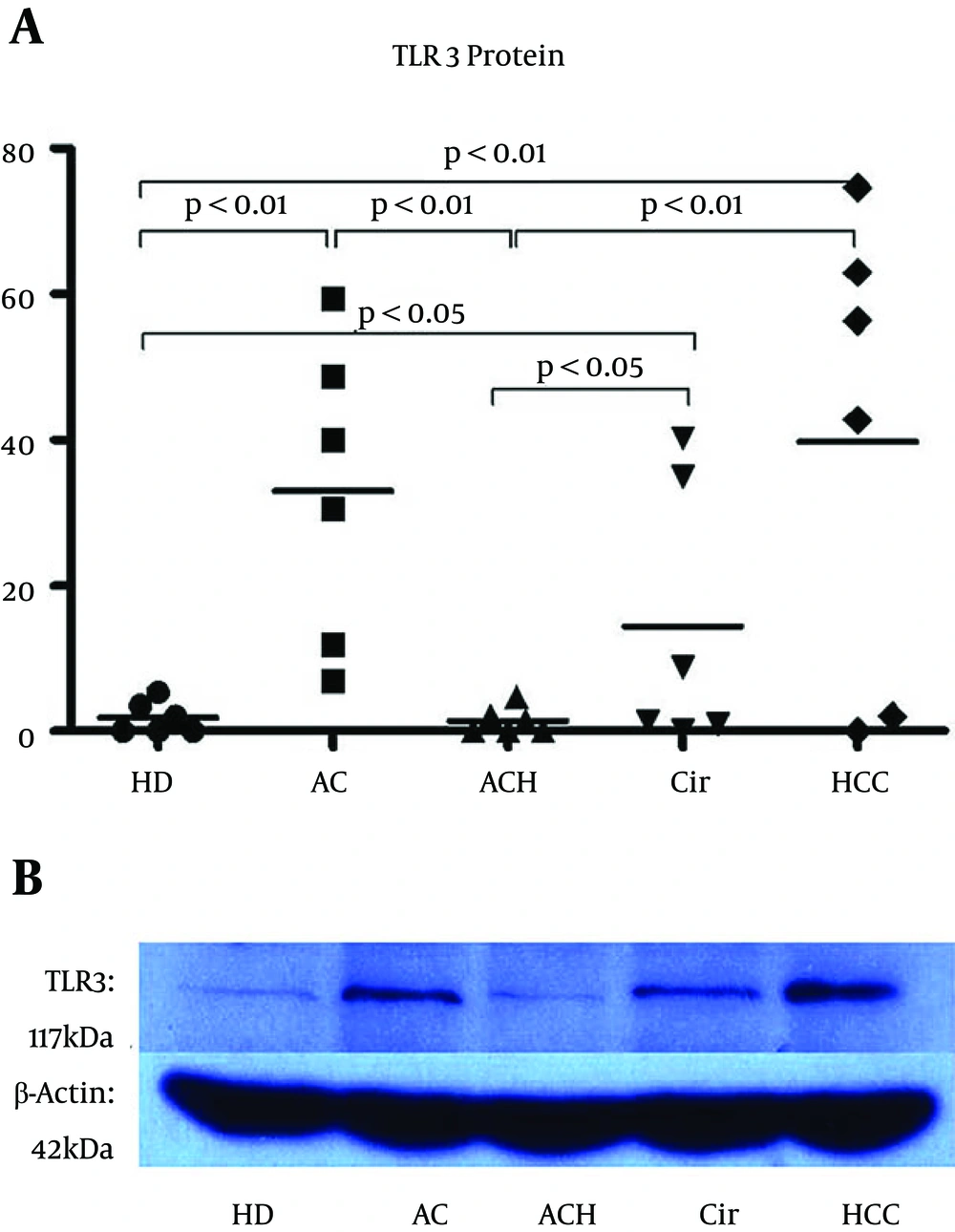
TLR3 is localized in the endosomes of hepatic NPCs, and minimally present in hepatocytes. Quantitative detection of the TLR3 protein in the liver using western blot analysis determined the immune state of the NPCs. Figure 1B illustrates a representative western blot analysis of TLR3 proteins in the livers of patients from six separate experiments. The TLR3 stains from HCC, AC, or Cir patients were darker than those from HD or ACH patients. The TLR3 levels in the HD, AC, ACH, Cir, and HCC groups were 1.82, 32.98, 1.36, 14.38, and 39.80, respectively (Figure 1A). The data showed that TLR3 expression in the ACH group tended toward a reduction, although the P Value obtained from comparing this group with the HD group was not statistically significant, and that TLR3 expressions in the HCC, AC, and Cir groups were higher than those in the HD and ACH groups.
A, Relative quantity of TLR3 proteins in the livers from all five groups (n = 6, in each group). The images on x-ray membranes were scanned, and the relative quantity of protein was normalized to the protein quantity for each sample using β-actin protein. All the parameters are shown. Significance was defined as P < 0.05, and extreme significance was defined as P < 0.01. B, Representative western blot analysis of TLR3 proteins in the livers from six separate experiments. Homogenates obtained from the livers of patients from the five groups were subjected to electrophoresis. β-actin protein served as a protein loading standard. The molecular weight of TLR3 and β-actin was 117 kDa and 42 kDa, respectively. The TLR3 stains in the HCC, AC, and Cir patients were darker than those in the HDs or ACH subjects.
4.3. Distribution of TLR3 in the Liver
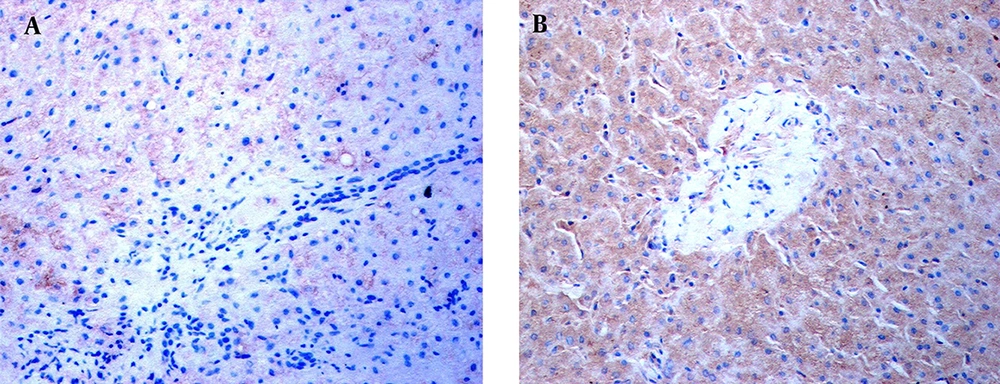
Immunohistochemical staining revealed the location of TLR3 in patients’ livers. In the livers of the ACH patients, weak TLR3 stains appeared on the hepatic sinusoid but no TLR3 stains were shown in the inflammatory-necrotic zone (Figure 2A). In AC, HCC, and Cir patients, TLR3 stains were observed in hepatocytes and NPCs (Figure 2B).
Immunohistochemical staining was performed using a specific antibody against human the TLR3 protein. TLR3 stains in the livers of an ACH patient and HCC patient are shown. A, No TLR3 stains were found in the NPCs, hepatocytes, or necroinflammatory zone in the liver of the ACH patient at × 400 magnification. B, TLR3 stains with brown granules primarily appeared in the NPCs and hepatocytes, with some staining exhibited in the cells of the portal area, in the HCC subject. The NPCs with TLR3 stains surrounded the hepatocytes at × 400 magnification.
4.4. Correlations Between TLR3 and Costimulatory Proteins in all Five Groups
To identify the effects of TLR3 on inflammatory reaction in CHB patients, the correlation coefficient between TLR3 and costimulation proteins was determined. The ACH, Cir, and HCC groups showed no correlation between TLR3 and all costimulatory proteins, including CD80, CD83, CD86, CD28, CTLA-4, CD40, and ICAM-1. In the AC group, TLR3 was negatively correlated with CD40 (P < 0.05), and it did not correlate with CD80, CD83, CD86, CD28, CTLA-4, and ICAM-1. Interestingly, TLR3 was positively correlated with ICAM-1 (P < 0.05) but did not correlate with CD80, CD83, CD86, CD28, CTLA-4, and CD40 in the HD group (Table 3). Overall, TLR3 was not interrelated with any costimulation proteins in DCs and T cells in all five groups. TLR3 was positively correlated with ICAM-1 in the HD group. No group, except the AC group, presented any interaction between TLR3 and CD40.
| TLR3 | CD80 | CD86 | CD83 | CD28 | CTLA-4 | CD40 | ICAM-1 |
|---|---|---|---|---|---|---|---|
| HD group | 0.792, 0.06 | -0.329, 0.525 | 0.491, 0.322 | -0.55, 0.258 | 0.793, 0.06 | -0.591, 0.216 | 0.83a, 0.041 |
| AC group | -0.612, 0.197 | 0.203, 0.699 | 0.177, 0.737 | -0.77, 0.073 | 0.794, 0.059 | -0.88a, 0.021 | -0.162, 0.759 |
| ACH group | 0.629, 0.181 | 0.496, 0.317 | -0.11, 0.983 | -0.337, 0.514 | 0.164, 0.756 | -0.486, 0.328 | 0.432, 0.329 |
| Cir group | -0.226, 0.667 | 0.735, 0.096 | -0.305, 0.556 | -0.084, 0.874 | -0.203, 0.7 | -0.524, 0.286 | 0.175, 0.74 |
| HCC group | 0.242, 0.644 | -0.038, 0.943 | 0.773, 0.072 | 0.259, 0.62 | 0.726, 0.103 | -0.277, 0.595 | 0.297, 0.567 |
Pairwise Correlation Between TLR3 and Costimulatory Proteins
5. Discussion
TLR3 signaling activation induces production of IFN-β and cytokines and contributes to HBV clearance. Contrarily, HBV components are able to block TLR3/IFN-β signaling and to counteract IFN-β responses through positive feedback loops (4, 6, 7). The data in the present study show that compared to the HDs, TLR3 in the livers of ACH patients was reduced and that intrahepatic TLR3 in HCC, AC, and Cir patients was increased, compared to HD and ACH subjects. This is the first study to report on the expression of TLR3 in the livers of patients with entire disease spectrum of CHB and reveal the interaction between TLR3 and the costimulation profile.
There were two problems with this study: (1) TLR3 levels in the AC, HCC, and Cir groups were enhanced compared to the ACH and HD groups, and (2) TLR3 levels were reduced in the ACH group, compared to the HD group. Previous studies showed that HBsAg, HBeAg, and HBV particles might inhibit the activation of NPCs through TLR3 ligands (4, 9). Additionally, TLR3 signaling molecules (i.e., TRIF, TRAF3, IRF3, and IRF7) presented lower expression levels in ACH patients compared to HDs in studies conducted by Momeni et al. and Ayoobi et al. (8, 10). In this study, the AC patients showed high viral loads positive for HBeAg and negative for intrahepatic hepatitis and having normal ALT levels, increased inhibitory costimulatory factors, and decreased inflammatory costimulatory proteins. The HCC patients displayed decreased expressions of both inhibitory and inflammatory costimulatory factors, and the Cir patients presented increased expressions of both inhibitory and inflammatory costimulatory factors. The ACH patients exhibited elevated necroinflammation scores, increased inflammatory costimulatory proteins, decreased inhibitory costimulatory proteins in the liver, and increased ALT levels in plasma. The above data imply that the degree of inflammatory activation progressively increased, but the ability of the HBV to inhibit inflammatory responses was gradually decreased in the AC, HCC, Cir, and ACH groups (3). The inhibition of HBV components was particularly important in the AC, HCC, and Cir groups, and interestingly, TLR3 levels in the livers of the AC, HCC, and Cir groups were increased.
Previous research studied feedback regulation in TLRs signaling pathways, in which positive/negative feedback regulation controlled the expression of up/down-stream factors and regulated immune and inflammatory reactions (21-23). Imaizumi et al. found that the positive feedback of IFN-stimulated gene56 regulated the expression of IFN-stimulated gene54 in the TLR3/IFN-β signaling pathway (23). Therefore, we assumed that compared to activation of TLR3/INF-β signaling, inhibition of TLR3/INF-β signaling by HBV components was predominant in AC, HCC, and Cir patients, and rich HBV components in the liver strongly suppressed the expression of TRIF, TRAF3, IRF3, and IRF7. Decreases in the level of TRIF, the molecule closest to TLR3, regulated the expression of TLR3 through positive feedback loops and induced high expression of TLR3 in the AC, HCC, and Cir subjects.
In previous reports, the reduction of TLR3 in ACH patients was identified through clinical observations (8, 10, 11). Huang’s study revealed that compared to HDs, ACH patients showed reduced levels of TLR3 in PBMCs before antiviral therapy, and patients who underwent interferon treatment show significantly restored levels of TLR3 (11). In the present study, TLR3 expression was detected in the liver of all patients, with the lowest levels found in ACH patients, followed by HD patients, which agreed with Huang’s data. Erdinest et al. reported that when poly I:C stimulated human corneal epithelial cells, TLR3 proteins in the cells were decreased, but the inflammatory cytokines were increased. They postulated that TLR3 ligands bound to receptors initiated the activation of the signal transduction pathway to coordinate cytokines responses, and subsequently over-activated downstream factors down-regulated TLR3 expression through negative feedback loops (24). The ACH patients in the present study had elevated necroinflammation scores in their livers and increased ALT levels in their plasma, which can signal inflammatory activation in the liver. The researchers speculated that activation of TLR3/IFN-β signaling promoted inflammatory reactions in the ACH group, while increased down-stream molecules strongly inhibited the expression of TLR3 by regulating the feedback loops, leading to minimal expression of TLR3.
Three clinical studies displayed different TLR3 expressions between ACH patients and HDs, including reduced TLR3 expression in the PBMCs of ACH patients in a study by Huang et al., increased TLR3 expression in patients with active stages of CHB and CHB-related liver failure in a study by Wang et al., and decreased TLR3 expression in the monocyte-derived dendritic cells of patients with ACH or acute-on-chronic liver failure in a study by Li et al. (8, 11, 12, 25). Ma et al. reported, based on unpublished results, that TLR expression and function might significantly change during the different phases of CHB (7). The findings in the present study are consistent with the results of Huang et al. (11) and Li et al. (25) but contradict the work of Wang et al. (12). The liver is an immune tolerant organ, in which apoptosis and degeneration of functional immune cells take place, resulting in intrahepatic immune suppression (14). TLR3 in the liver, instead of peripheral blood, exhibits the actual immune status of CHB. Li et al. (25) and Wang et al. (12) detected only TLR3 expression in PBMCs, while Huang et al. (11) and the present study showed that TLR3 expression in the liver of CHB patients can reveal the actual immune status of the liver. This study in particular used the entire disease spectrum of CHB, including AC, ACH, Cir, and HCC, and is applicable for investigating the interaction between TLR3 and costimulation proteins. Here, increased TLR3 proteins in the livers of AC, HCC, and Cir patients was reported for the first time, and TLR3 proteins presented significant differences in the AC, ACH, Cir, and HCC groups, which agrees with Ma et al.’s findings (7). Additionally, this study’s data revealed the partial characteristics of immune responses to CHB.
Costimulatory proteins in the liver was quantitatively detected, including CD80, CD86, CD83, CD28, CTLA-4, CD40, and ICAM-1 (3). The surface of DCs presented CD80, CD86, and CD83. Maturated DCs showed increased CD80, CD86, and CD83 (14, 26), resulting in activation of T cells and contributing to immune responses in the liver (7, 11). CD28 and CTLA-4 are attached to the surface of T cells, and increased CD28 implied activation of the T cells. Conversely, increased CTLA-4 indicated inhibition of T cells (27). CD40 was expressed on the surface of immune cells or non-immune cells, and increased CD40 implied that the CD40+ cells proliferated and increased inflammatory reactions (28). ICAM-1 was secreted by CD40+ cells and participated in adhesion among various immune cells (29).
In the present study, TLR3 did not influence maturated DCs or activated T cell signals in any of the five groups (Table 3); however, the mechanisms for this remain unknown. Additionally, a negative correlation between TLR3 and CD40 was present in the AC group, but no correlation between TLR3 with CD40 was found in the ACH, Cir, or HCC groups. While detecting costimulation proteins, decreased CD40 was found and associated with immune tolerance in the AC group (3). These results indicated that HBV components inhibited inflammatory responses in NPCs and hepatocytes, resulting in increased TLR3 and decreased CD40 in AC patients. Additionally, it is presumed that imbalances between HBV components and host immune responses during the immune tolerance phase change the levels of costimulation proteins that induce various immune statuses in ACH, Cir, and HCC patients. Subsequently, the interaction between TLR3 and CD40 deteriorated. Finally, TLR3 was positively correlated with ICAM-1 in the HD group, and it was previously reported that the levels of ICAM-1 were the lowest in the HD group compared to the other four groups (3). Therefore, a positive correlation between TLR3 and ICAM-1 in HDs who did not exhibit HBV immune responses in the liver is irrelevant. In short, TLR3/INF-β signaling did not influence the expression of costimulatory proteins in AC, ACH, Cir, or HCC patients, although the AC group exhibited a negative correlation between TLR3 and CD40.
In conclusion, the AC, HCC, and Cir patients in the present study displayed increased TLR3 proteins in their livers, while the ACH patients exhibited reduced TLR3. This work suggests that both activation of TLR3/INF-β signaling and inhibition of TLR3/INF-β signaling by HBV components influence TLR3 expression in AC, ACH, Cir, and HCC subjects. However, TLR3/INF-β signaling does not influence the expression of costimulatory proteins in ACH, Cir, or HCC patients.