1. Background
Interleukin (IL)-7 belongs to the IL-2 cytokine family (1). IL-7 is mainly a glycoprotein secreted by matrix cells (bone marrow, thymus, soft tissue) (2, 3), epithelial cells (liver and intestine) (4, 5), endothelial cells (6), fibroblasts (4), keratinocytes (7), and dendritic cells (8). IL-7 can stimulate immature and memory lymphocytes to proliferate; it is one of the regulatory cytokines for the balance of peripheral T cells (9, 10). In chronic viral infectious diseases, IL-7 may increase the ability of virus-specific T cells to clear the virus (11), and in animals treated with combined IL-7 and IL-21, the anti-HBV-specific T cells apparently amplify, which produces interferon-γ and reduces the level of viremia (12). Seo et al. reported (13) that IL-7 plays a pivotal role in the generation of T follicular helper (Tfh) cells and found that exogenous IL-7 could enhance immature T cells to differentiate to Tfh cells. Although the definitive mechanism by which IL-7 upregulates the production of Tfh cells is not yet known, we are inspired to understand that in addition to helping B lymphocytes, Tfh cells can also secrete IL-21 (14), and IL-21 can promote the proliferation of virus-specific cytotoxic T lymphocytes (CTL) (15, 16). Therefore, Tfh cells have an influence on cellular immunity. Our previous studies on chronic hepatitis B patients showed that Tfh cells increased the levels of hepatitis B virus-specific CTL through IL-21 and thereby decreased the levels of HBV DNA (17), while IL-7 is related to Tfh cells.
2. Objectives
We studied the relationship of peripheral blood IL-7 in CHB patients, along with the levels of Tfh cells, IL-21, HBV-specific CTL, and HBV DNA, to explore whether peripheral blood IL-7 of CHB patients has any influence on the levels of Tfh cells and HBV-specific CTL. This was done to provide evidence for further study of the implications of IL-7 on the cellular immunity of CHB patients and on the treatment of CHB with IL-7.
3. Methods
3.1. Patients
Ninety-one patients with CHB were enrolled into this study from August 2013 to March 2015 in the Wuxi area, according to the following inclusion and exclusion criteria. Inclusion criteria: all these patients’ diagnoses had to meet the diagnostic criteria for chronic hepatitis B of the 2010 Chinese guidelines for prevention and treatment of chronic Hepatitis B (18): HBV DNA positive (HBV DNA ≥ 102 copies/mL), hepatitis B e antigen (HBeAg)-positive, human leukocyte antigen (HLA)-A2-positive, alanine aminotransferase (ALT) > 2 × upper limit of normal range, and adults of an age ≥ 18 years. Exclusion criteria: patients who had concomitant infection with type A, C, D, or E hepatitis virus; patients with a history of autoimmune hepatitis or other autoimmune disorders, with a history of alcohol addiction, using hepatotoxic drugs, or receiving treatment with such antiviral agents as nucleos(t)ide analogues and/or interferons or immunomodulatory drugs.
This study was approved by the medical ethics committee of Jiangnan University (No.2013050509), and every patient signed the written informed consent.
The 91 patients were divided into three groups (A, B, and C) according to their levels of IL-7 (low, medium, or high). Group A had a low IL-7 level, ≤ 22 (ng/L), group B had a medium IL-7 level of 23 - 28 (ng/L), and group C had high IL-7 level, ≥ 29 (ng/L). The levels of IL-7 of the three groups were significantly different.
3.2. Monoclonal Antibodies
The Beckman-Coulter company supplied the following antibodies: CD4 FITC (RPT-T4), CD19 FITC (HIB19), CD3 PC-5 (UCHT1), CD8 FITC (RTP-T8), HLA-A2 FITC (BB7.2), and MS IGG1 KPA ITCL FITC (MOPC-21).
3.3. Determination of Peripheral Blood IL-7 and IL-21
A double antibody sandwich enzyme-linked immunosorbent assay was used for the purpose of determining the peripheral blood IL-7 and IL-21. The major device used included an enzyme immunoassay reader/analyzer (HY-2511, produced by Delang Co. Ltd., Wuxi, China) and a reagent kit (ADL Co. Ltd., San Diego, CA, USA). We performed the determination procedures according to the instructions for use provided by the manufacturers.
3.4. Determination of Peripheral Blood Tfh Cells
To determine the peripheral blood Tfh cells, 10 μL of CXCR5 monoclonal antibody was added into 100 μL heparin anticoagulated whole blood and incubated at 4 for 30 minutes, and then 10 μL of monoclonal antibodies against CD4 and CD19 was added at the same time; the corresponding control monoclonal antibodies were added to the control tubes and were incubated at 4 for 30 minutes. After hemolysis and washing with an FACS washing solution, the cells were fixed with an FACS fixing solution. The reagents of the CXCR5 monoclonal antibody and the corresponding control monoclonal antibodies were from Biolegend, and the reagents for other items were from Beckman-Coulter. A flow cytometer (Beckman-Coulter FC500, Miami, Florida, USA) was used for the determination. Data were collected and analyzed with the software package CXP.
3.5. Identification of HLA-A2 Allele Type
Into the test tube and the control tube was added 100 μL of heparin anticoagulated fresh whole blood specimen, and HLA-A2-phycoerythrin and 10 μL of the same type of control (MS IGG1 KPA ITCL FITC (MOPC-21)), respectively, were also added into the test tube and the control tube and were incubated at room temperature in the dark for 30 minutes. After hemolysis, a flow cytometer was used to determine. The reagents were from Beckman-Coulter.
3.6. Determination of HBV-Specific CTL
Into a tube were placed 10 μL of an HLA-peptide tetramer labeled with phycoerythrin and CD8-fluorescin isothiocyanate, CD3-PC5, and 1 μL HBV core 18 - 27 antigen peptide; then 100 μL heparin anticoagulated blood was added and at the same time, the same type of control (R-PE-labelled Pro5® Recombinant HLA-A*0201-negative control tetramer). After being mixed well, the test tubes with the mixtures were incubated at room temperature for 20 minutes. After hemolysis and washing, detection was performed by using a flow cytometer. After the lymphocyte counting gate was set with CD3+ lymphocytes, 50,000 CD8+ cells were counted, and CD8+ and HLA-peptide tetramer double positive cells were regarded as HBV-specific CD8+ T lymphocytes and were expressed as a percentage of the total count of CD8+ cells. The reagents for the HBV core 18 - 27 antigen peptide and the R-PE labelled Pro5® Recombinant HLA-A*0201-negative control tetramer were from Proimmune, UK, and the other items were from Beckman-Coulter.
3.7. Detection of HBV Markers and HBV DNA
The Hepatitis B surface antigen (HBsAg), the antibody against HBsAg (anti-HBs), HBeAg, the antibody to HBeAg (anti-HBe), and the antibody to the hepatitis B core antigen (HBcAg) (anti-HBc) were detected with a time-resolved fluorescence method using reagents from Shanghai Xinbo biotechnological corporation Ltd., China. The HBV DNA was determined by using a real-time fluorescence quantitative polymerase chain reaction (PCR) with reagents produced by Shanghai Kehua Bioengineering corporation, Ltd., China.
3.8. Liver Function Tests
A Hitachi 7600 fully automated biochemical analyzer was used to detect serum ALT, total bilirubin, and albumin.
3.9. Statistical Methods
Analyses of the data were performed by using the software SPSS 12.0; categorical data were compared with the chi-square test; normally distributed continuous data were expressed as mean ± standard deviation (SD) and were compared with a t-test for two samples and with an analysis of variance for more than two groups. Correlation between different factors was studied with a Pearson correlation analysis, and the analysis of multiple factors was performed by using multiple linear regression. P < 0.05 was regarded as having statistical significance.
4. Results
4.1. Age and Sex of the 91 Patients With CHB and 30 Healthy Controls
Of the 91 patients, 65 were male (71.43%) and 26 female (28.57%); the mean age of the patients was 36.14 ± 11.16 years. Of the 30 healthy controls, 22 (73.33%) were male and 8 (26.67%) were female, and the mean age was 36.03 ± 7.84 years. Comparison of age, sex and course of disease of the patients in groups A, B, and C is shown in Table 1.
| Group | No. of Cases | IL-7, ng/L | Male/Female | Age, y (Mean ± SD) | Course of Disease, y (Mean ± SD) |
|---|---|---|---|---|---|
| A | 31 | 20.85 ± 5.24 | 22/9 | 36.10 ± 8.20 | 5.06 ± 2.98 |
| B | 30 | 25.89 ± 7.55 | 21/9 | 36.07 ± 12.97 | 4.63 ± 2.74 |
| C | 30 | 30.34 ± 3.05 | 22/8 | 36.27 ± 12.20 | 4.53 ± 2.96 |
| F/χ2 Value | 22.050 | χ2 = 0.087 | 0.003 | 0.292 | |
| P Value | < 0.01 | > 0.05 | > 0.05 | > 0.05 |
4.2. Comparison of Peripheral Blood Levels of IL-7, Tfh Cells, IL-21, HBV-Specific CTL, and HBV DNA Among Groups A, B, and C and Between Patient Group and Healthy Control Group
The mean level of IL-7 for the 91 cases with CHB (25.64 ± 6.77 ng/L) was significantly lower than that of the healthy control group (45.23 ± 9.79 ng/L), t = 12.22, P < 0.01; the level of Tfh cells of the CHB group (4.83 ± 1.64%) was significantly higher than that of the healthy control group (2.65 ± 0.63%), t = 10.53, P < 0.01. The level of IL-21 of the CHB group (30.79 ± 8.72 ng/L) was significantly lower than that of the healthy control group (70.30 ± 10.69 ng/L), t = 20.308, P < 0.01.
Table 2 shows the results of determinations of the levels of IL-7, Tfh cells, IL-21, HBV-specific CTL, and HBV DNA for groups A, B, and C.
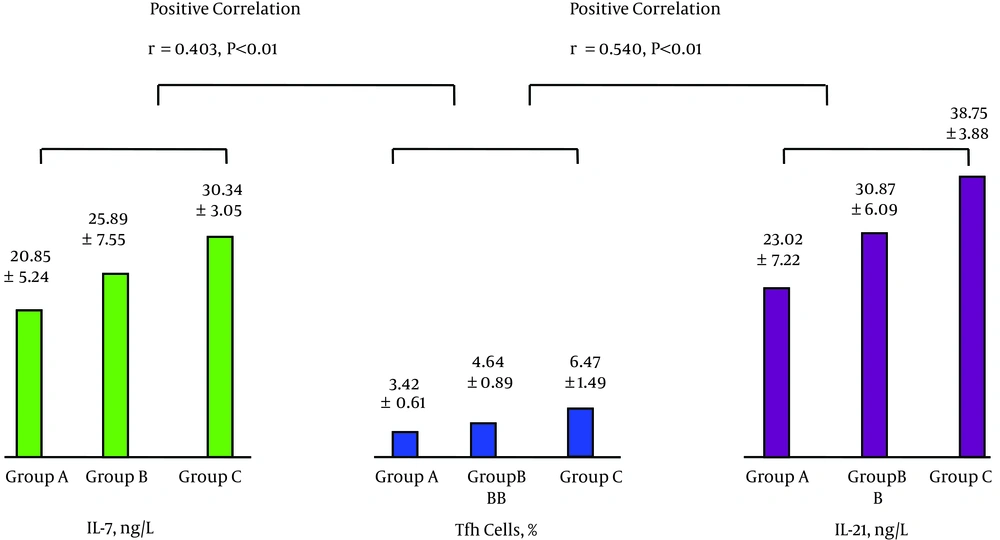
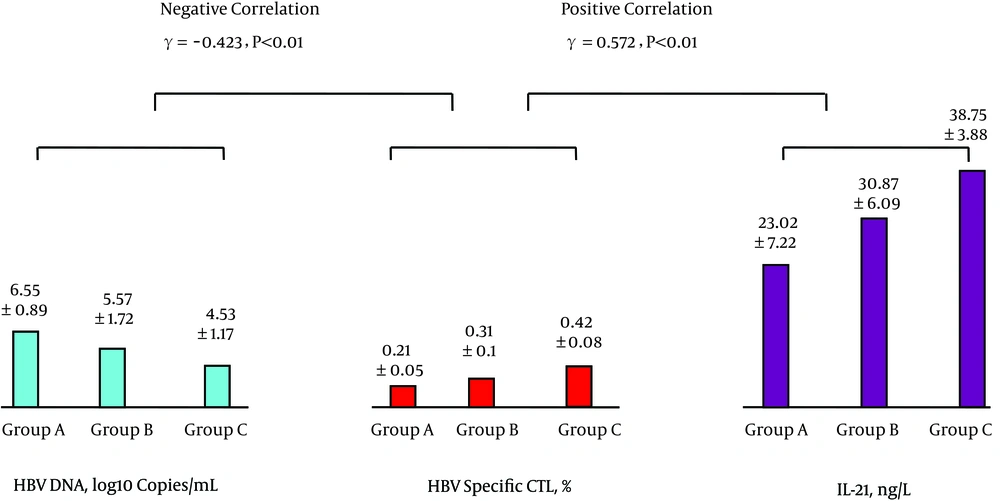
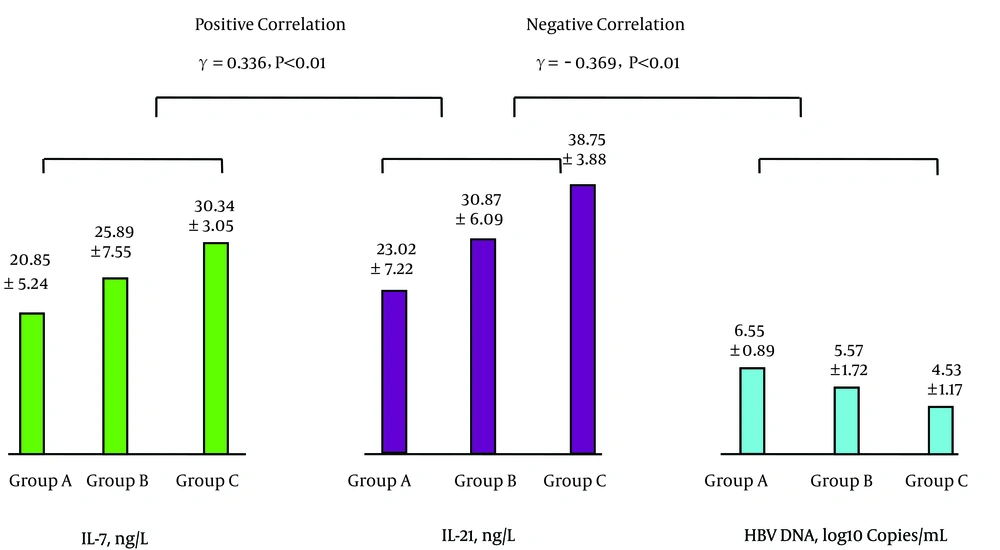
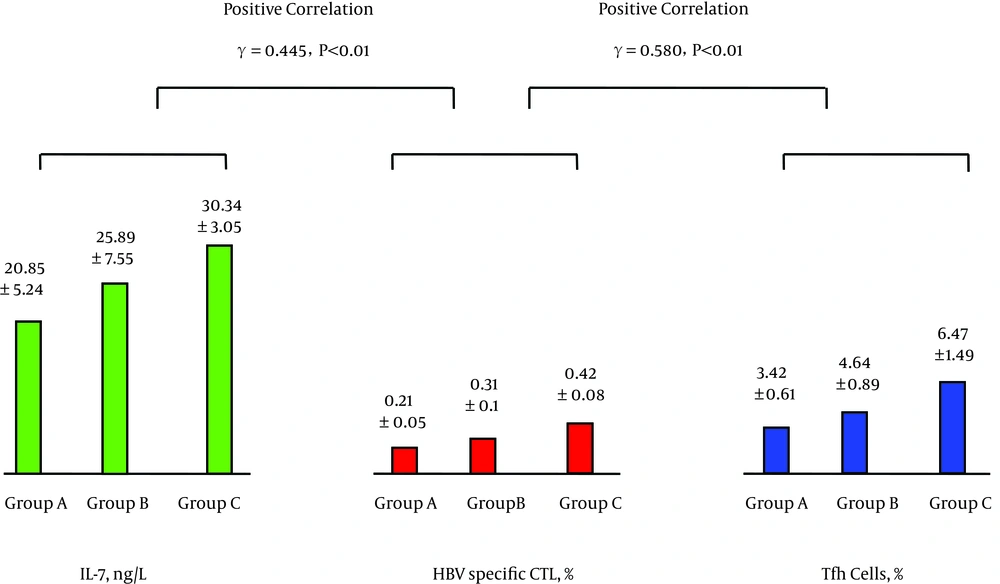
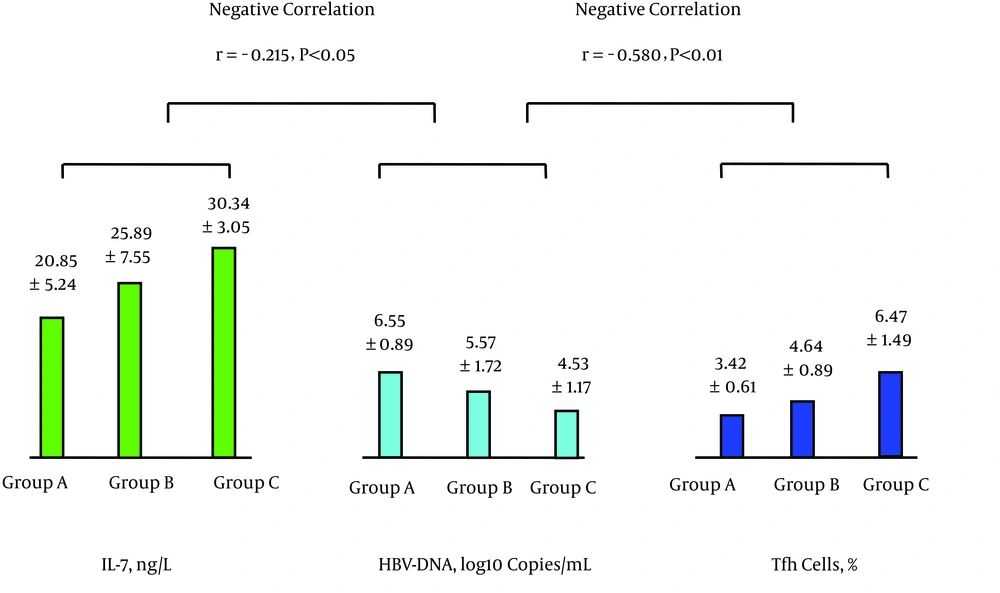
| Group | No. of Cases | IL-7, ng/L | Tfh Cells, % | IL-21, ng/L | HBV-Specific CTL, % | HBV DNA, Log10 Copies/mL |
|---|---|---|---|---|---|---|
| A | 31 | 20.85 ± 5.24 | 3.42 ± 0.61 | 23.02 ± 7.22 | 0.21 ± 0.05 | 6.55 ± 0.89 |
| B | 30 | 25.89 ± 7.55a | 4.64 ± 0.89A | 30.87 ± 6.09A | 0.31 ± 0.1A | 5.57 ± 1.72A1 |
| C | 30 | 30.34 ± 3.05AB | 6.47 ± 1.49AB | 38.75 ± 3.88AB | 0.42 ± 0.08AB | 4.53 ± 1.17AB |
| F Value | 22.050 | 64.022 | 53.886 | 58.245 | 18.341 | |
| P Value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Abbreviations: CTL, cytotoxic T lymphocytes; HBV, hepatitis B virus; IL, interleukin; Tfh cells, T follicular helper.
aA, P < 0.01 compared with group A; A1, P < 0.05 compared with group A; B, P < 0.01 compared with group B.
4.3. Analyses of Correlation Between Levels of Peripheral Blood IL-7 and Tfh Cells, Tfh Cells and IL-21, IL-21 and HBV-Specific CTL, HBV-Specific CTL and HBV DNA
The levels of peripheral blood IL-7 and Tfh cells were positively correlated as were the levels of Tfh cells and IL-21; see Figure 1. The levels of IL-21 and HBV-specific CTL were positively correlated, while the levels of HBV-specific CTL and HBV DNA were negatively correlated; see Figure 2. The levels of IL-7 and IL-21 were positively correlated, while the levels of IL-21 and HBV DNA were negatively correlated; see Figure 3. The levels of IL-7 and HBV-specific CTL were positively correlated as were the levels of HBV-specific CTL and Tfh cells; see Figure 4. The levels of IL-7 and HBV DNA were negatively correlated, and the levels of HBV DNA and Tfh cells were also negatively correlated; see Figure 5. Multiple linear regression analysis is shown in Table 3.
| Factor | Unstandardized Coefficients | Standard Error | Standardized Coefficients | T Value | P Value | 95% Confidence Interval for B |
|---|---|---|---|---|---|---|
| B | Beta | |||||
| HBV-specific CTLa | -5.558 | 1.264 | -0.423 | -4.398 | < 0.01 | -8.069 - -3.047 |
| IL-7b | 3.532E-03 | 0.001 | 0.206 | 2.373 | < 0.05 | 0.001 - 0.006 |
| IL-21b | 4.419E-03 | 0.001 | 0.332 | 3.521 | < 0.01 | 0.002 - 0.007 |
| Tfh cellsb | 2.258E-02 | 0.007 | 0.318 | 3.281 | < 0.01 | 0.009 - 0.036 |
| Tfh cellsc | 2.874 | 0.475 | 0.540 | 6.048 | < 0.01 | 1.930 - 3.819 |
aEffects of IL-7, Tfh cells, IL-21, and HBV-specific CTL on HBV DNA (dependent variable).
bEffects of IL-7, IL-21, and Tfh cells on HBV-specific CTL (dependent variable).
cEffects of IL-7 and Tfh cells on IL-21 (dependent variable).
5. Discussion
In patients with chronic hepatitis B, the capability of clearing the HBV is related to the cellular immune function of the body. One of the current directions of research on CHB is to find a therapeutic approach that can enhance patients’ cellular immune function and improve the effect of treatment for CHB, thereby improving the patients’ prognosis. Studies have shown that injection of exogenous IL-7 into mice could result in a significant increase of T and B lymphocytes; clinically, subcutaneous injection with IL-7 could also promote proliferation of T and B lymphocytes and could effectively maintain T cell internal homeostasis (19-21). IL-7 can increase the activity of CTL (22). IL-7 plays an important role in the normal development of the immune system and maintenance of immune functions, and its biological effect is produced mainly through binding to the receptor α of IL-7 (IL-7Rα) (23, 24). Recently, Seo et al. (13) reported for the first time that IL-7 plays a pivotal role in the generation of Tfh cells and the induction of humoral immunity; exogenous IL-7 can enhance the immature T cells of mice to differentiate to functional Tfh cells. This discovery provides novel thinking pathways for studies on the implications of IL-7 on the cellular immune functions of patients with chronic viral infections such as chronic HBV infection. In the present study, we investigated the relationship of the levels of IL-7 with Tfh cells and HBV-specific CTL in CHB patients. We explored to see whether IL-7 could increase the level of Tfh cells and if so, to understand the clinical significance thereof. This study showed that in CHB patients, the level of IL-7 was lower than that of normal persons. We divided the CHB patients into groups A, B, and C, according to their IL-7 levels in the order of lower to higher. We studied the relationship between the levels of IL-7 and Tfh cells and their implications on HBV-specific cellular immune function, and we found that the levels of Tfh cells increased with the levels of IL-7 (correlated positively), which indicates that the IL-7 of CHB patients possibly enhances Tfh cell production and that Tfh cells can influence cellular immunity. Feng et al.’s study showed that CHB patients having active immune responses had significantly more peripheral blood Tfh cells as compared to CHB patients who had already developed immune tolerance. Based on this finding, it can be speculated that Tfh cells possibly participate in the immune responses of CHB patients (25) and that in chronic hepatitis C (CHC) patients, an increased number of Tfh cells may help to control the viral infection (26). Tfh cells probably play an antiviral role via secreting IL-21. Tfh cells can secrete IL-21 (27, 28), and IL-21 can enhance the proliferation of virus-specific CTL (15, 16). Specific CTL-mediated immune responses against viruses like HBV play very important roles in clearing the viruses (29-32). Therefore, the HBV-specific CTL levels of CHB patients are closely related to HBV DNA levels; those who have low levels of HBV-specific CTL have high HBV DNA levels (33, 34), and the relationship between virus-specific CTL levels and virus DNA levels is similar to the results of studies on chronic HCV- and HIV-infected persons (35-37).
In the present study, it was demonstrated that the levels of Tfh cells, IL-21, and HBV-specific CTL increased with the elevation of IL-7 in CHB patients (positive correlations were found among the levels of IL-7, Tfh cells, IL-21, and HBV-specific CTL), but the level of HBV DNA decreased (IL-7, Tfh cells, IL-21, and HBV-specific CTL levels were all negatively correlated with HBV DNA levels). Therefore, in CHB patients, IL-7 may have promoted specific cellular immune responses through increasing the levels of Tfh cells, which are beneficial to the inhibition or clearing of HBV.
Multiple linear regression analyses showed that HBV-specific CTL is an independent factor influencing HBV DNA and that IL-7, Tfh cells, and IL-21 are all independent factors that influence HBV-specific CTL. IL-7, Tfh cells, and IL-21 may increase the levels of HBV-specific CTL through their effects on HBV-specific CTL to decrease the level of HBV DNA. The mechanism by which the Tfh cells independently influence the HBV-specific CTL is not clear. Tfh cells can independently influence IL-21, as reported by researchers who showed that Tfh cells can secrete IL-21 (27, 28), while IL-21 may promote Tfh cells to differentiate and develop (28, 38); a benign circulation forms between the Tfh cells and IL-21 that is beneficial to increasing the level of HBV-specific CTL. These interactions suggest that in addition to directly increasing the HBV-specific CTL level, IL-7 in patients with CHB possibly increases the level of the Tfh cells. In turn, these cells, besides possibly directly increasing the level of HBV-specific CTL, may also increase the level of HBV-specific CTL via increasing the level of IL-21.
In summary, the present study showed in a preliminary way that in patients with CHB, the levels of IL-7 were related to the levels of Tfh cells (positively correlated); the higher the level of IL-7, the higher the levels of Tfh cells, IL-21, and HBV-specific CTL and the lower the levels of HBV DNA. IL-7 in CHB patients may increase the level of Tfh cells, and Tfh cells may increase virus-specific cellular immune responses. IL-7 may also directly increase the level of HBV-specific CTL and promote specific cellular immune responses beneficial to the elimination or inhibition of HBV. This study provides evidence for the possibility of treating CHB patients with IL-7, which may improve CHB patients’ virus-specific cellular immune functions and thereby inhibit or clear HBV.
A limitation of the present study is the fact that we have not studied the mechanisms of how IL-7 increases the levels of Tfh cells. Our future studies will include such mechanisms.