1. Background
Hepatitis A virus (HAV) infection is an international public health concern that results in considerable morbidity in both developing and developed countries. Humans are the only natural host for HAV, and the virus is excreted in high concentrations in the stool of infected patients. Therefore, this infection is mainly transmitted through the fecal-oral route via ingestion of contaminated foods or drinking water or by direct contact with an infected person (1).
The main geographical alterations in the prevalence of HAV infection are closely correlated with sanitary and hygienic conditions and other indicators of socioeconomic status. In areas of low socioeconomic status, HAV infection primarily occurs in childhood, when the infection is mostly asymptomatic and does not represent a public health problem. Conversely, in areas with high socioeconomic status, the infection of adults can bring substantial risk of mortality and morbidity (2).
Because the severity of HAV infection increases after six years of age, understanding the prevalence of this infection in children and adolescents in each geographic area is important to target the local interventions necessary for preventing, treating, and diagnosing the disease (1).
Iran is a country with intermediate endemicity for HAV infection. As previously reported, the overall rate of HAV seropositivity in Iran is 64%, which increases sharply from 14.8% at age 10 to 72.9% at age 13 (3). Iran is a vast country consisting of 31 provinces with differing sanitary conditions and socioeconomic properties, so the prevalence of this infection is expected to vary across various parts of the country. There is no surveillance program for monitoring HAV infection in different provinces of Iran, and seroprevalence studies are the main source of information about HAV infection (4).
The few studies that have assessed the seroprevalence of HAV infection in Iran have shown different results. In 2006, a multicenter study revealed that in the age group of 18-25 years, the seroprevalences of HAV in the Tehran, Hormozgan, and Golestan provinces located in central, southern, and northern Iran were 65%, 92%, and 99%, respectively. Factors associated with increased seropositivity were being married and fewer years of father’s education (5).
Another report in 2003 from Shahrekord city, central Iran found that in the 15–24-year-old population, the seroprevalence of anti-HAV was 90.8%. Education level, marital status, and ethnicity were associated with HAV seropositivity in the studied individuals (6). Moreover, HAV seroprevalence in 273 children aged 7-10 years in Zanjan city in 2002 was reported as 44.3%. No significant difference was found in terms of gender (7).
A cross-sectional study on 1,030 samples that were collected between 2011 and 2012 from schools, health centers, and outpatient clinics in Shiraz, southern Iran showed HAV seropositivities of 18.3% and 79.4% in the age groups of 6 - 15 and 16 - 29 years, respectively (8). Another study in Fars province, southern Iran conducted on 1,050 individuals attending pre-marriage classes indicated that 79.3% of the population in the age group of 15 - 20 years carried anti-HAV antibodies. The HAV seropositivity was significantly positively correlated with large family size (9). A study conducted on 252 children aged 2-16 years who attended the gastroenterology clinic of the Tabriz Children’s Hospital in northwest Iran during 2012 reported an HAV seropositivity rate of 32.9% (10).
Existing reports on HAV seroprevalence are limited to certain populations or regions and are not sufficient to inform policy making. This study aims to determine the prevalence of HAV infection in Iranian adolescents of different provinces and to investigate underlying risk factors for infection associated with household and provincial socioeconomic characteristics.
2. Objectives
As far as we know, no previous study has evaluated this many provinces in Iran. This national and consistent study will help policy makers efficiently implement HAV vaccination in Iran.
3. Methods
3.1. Population and Setting
In a nationwide cross-sectional study, serum samples from a national school-based survey called CASPIAN III (2009-2010) were tested for anti-HAV antibodies. Participants in the main study were 10 to 18-year-old students living in 27 provinces of Iran. Multistage random cluster sampling was used. In each province, cluster sampling with equal clusters was used to reach the necessary sample size, and schools were stratified and randomly selected from the information bank of the Ministry of Education. Stratification was performed according to residence area (urban/rural) with equal sex ratios, as previously explained (11). Blood samples were obtained from randomly selected students; subsample sera were stored at -70°C. Information regarding demographic and socioeconomic factors of participants were obtained by a validated questionnaire, which was filled in by the participants’ parents (11).
To estimate the prevalence of HAV infection in different provinces, a minimum sample size of about 90 was calculated for each province by assuming an expected prevalence of 40%, a confidence interval (CI) of 95%, and a precision of 10%. Serum samples and related databases were available from 17 provinces. All samples available from these 17 provinces were included in this study (2,562 serum samples); however, samples from 68 cases were not large enough for serologic assay because they were depleted in previous laboratory tests; these were excluded from the study.
3.2. Measurements
Anti-HAV antibodies (IgG and IgM) were measured using a competitive enzyme immunoassay kit (Dia.Pro, Italy) according to the kit instructions by a BioTek ELISA plate reader (Biotek Instruments, USA), which was calibrated with a check plate. A ratio of cut-off value to OD450 nm of the sample > 1.1 was considered positive, and uncertain results were repeated. The laboratory procedure was performed at the Infectious Diseases and Tropical Medicine Research Centre of the Isfahan University of Medical Sciences.
Household-level risk factors including number of household members, parents’ education level, parents’ job, and a number of socioeconomic factors, including whether participants’ families owned a personal home, car, or computer and whether or not participants attended a private school (combined into a single variable named socioeconomic status [SES]) were extracted from the main database (11). The 2011 National Population and Housing Census data (12) was the source of province-level risk factors, which were accessed using the following variables: having access to healthy drinking water (pipe water or bottled water), the ratio of family income to costs (household budget), and the population density (population/area[m2]) of each province.
3.3. Statistical Methods
The prevalence of HAV infection and the 95% CI for each province were calculated. Weighting was applied to correct the difference in sampling ratios between age groups and rural/urban residency, according to the definite proportions adopted from national census data for each province (12). The association between HAV seropositivity and independent variables was assessed by a multilevel analysis using mixed-effects logistic regression (melogit), and reported in terms of odds ratio (OR) and 95% CI. The analysis was performed using Stata 13 software (Stata Corp, College Station, TX), and a P-value of less than 0.05 was considered significant.
3.4. Ethical Approval
The CASPIAN-III study was approved by the ethical committees of relevant national and provincial organizations, and written informed consent was obtained from one of each of the participants’ parents for venipuncture and for use of their household information in research projects. The procedure was fully explained to the students, and participation in the project was voluntary (11). The ethical committee of the Isfahan University of Medical Sciences in Isfahan, Iran approved the current study (Project number: 293148-50).
4. Results
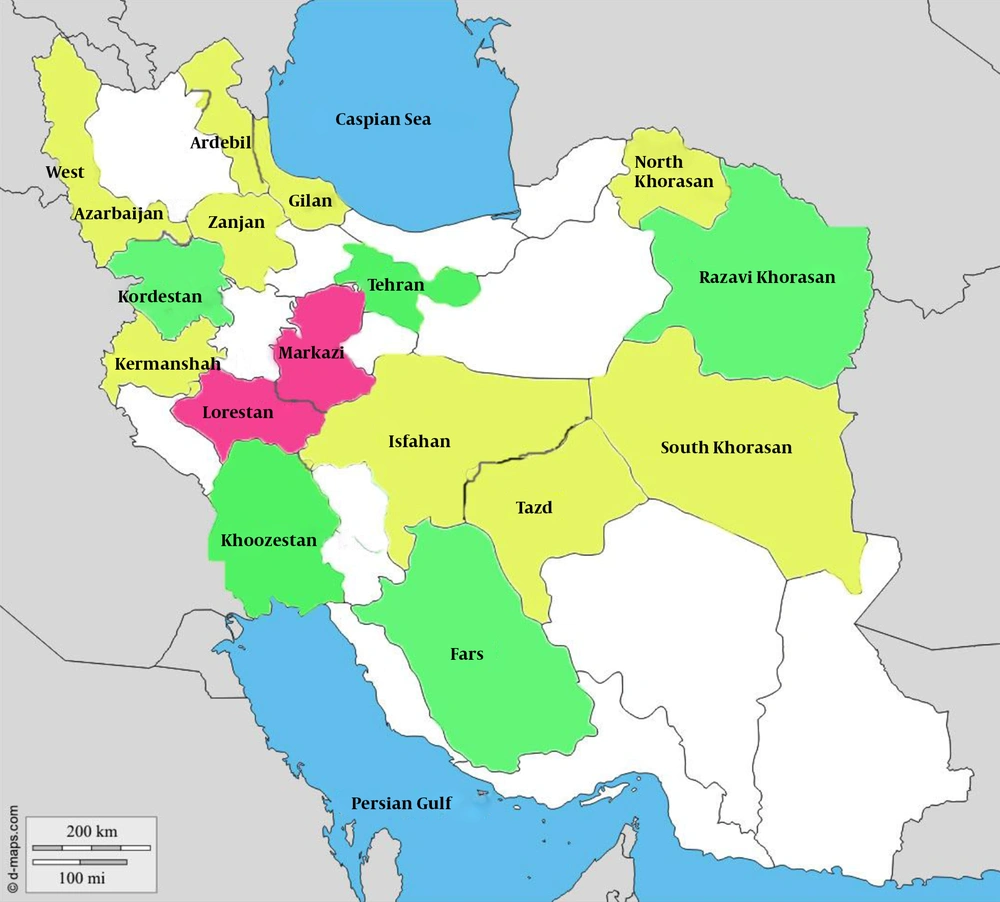
The weighted prevalence of HAV infection varied in a statistically significant manner across the 16 provinces of Iran that were surveyed (P = 0.001) (Table 1). As shown in Figure 1, the Fars and Kordestan provinces had the lowest prevalences (50.43% and 52.80%, respectively), whereas the Markazi and Lorestan provinces had the highest prevalences (78.81% and 73.74%, respectively). Because the sample sizes in the Chaharmahal and Bakhtiari provinces were less than 90, we could not report the true prevalence in these provinces; however, the cases from these provinces were included in the risk factor analysis.
| Province | Number | Corrected Prevalence (%) | 95% CIa |
|---|---|---|---|
| Ardabil | 205 | 67.63 | 61.10-74.09 |
| Azarbaijan, West | 142 | 67.267.29 | 59.48-75.10 |
| Fars | 100 | 50.43 | 40.46-60.40 |
| Gilan | 133 | 64.96 | 56.75-73.18 |
| Isfahan | 147 | 67.43 | 59.77-75.10 |
| Kermanshah | 231 | 61.59 | 55.27-67.91 |
| Khorasan, North | 120 | 64.29 | 55.59-72.99 |
| Khorasan, Razavi | 167 | 52.83 | 45.18-60.48 |
| Khorasan, South | 125 | 61.35 | 52.69-70.00 |
| Khoozestan | 195 | 59.46 | 52.51-66.41 |
| Kordestan | 145 | 52.80 | 44.57-61.02 |
| Lorestan | 146 | 73.74 | 66.52-80.97 |
| Markazi | 93 | 78.81 | 70.35-87.27 |
| Tehran | 250 | 57.90 | 51.74-64.06 |
| Yazd | 145 | 67.66 | 59.96-75.37 |
| Zanjan | 92 | 60.28 | 50.09-70.46 |
Hepatitis A Prevalence in 10 to 18-Year-Old Adolescents in 16 Provinces of Iran: the CASPIAN-III Study
Overall, 2,494 cases were analyzed for related risk factors. The intracluster correlation coefficient (ICC) for the multilevel model was 0.012. Table 2 presents the demographic-, household-, and province-level risk factors for HAV infection. Among the demographic variables, only age group was related to HAV prevalence; HAV prevalence was significantly higher in 13-15 and 16-18-year-olds than in 10-12-year-olds. About 84% of the participants were living with five or fewer household members. This number was not associated with the prevalence of HAV.
| Risk Factors | N Positive/Total (Percent) | OR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 788/1250 (63.00) | 1 | |||
| Female | 808/1241 (65.10) | 0.82 | 0.99 - 1.20 | 0.949 | |
| Residence | |||||
| Urban | 1045/1649 (63.40) | 1 | |||
| Rural | 550/843 (65.2) | 1.13 | 0.80 - 1.59 | 0.462 | |
| Age group | |||||
| 10 - 12 | 360/790 (45.60) | 1 | |||
| 13 - 15 | 421/585 (72.00) | 3.03 | 2.33 - 3.93 | 0.000 | |
| 16 - 18 | 816/1119 (72.9) | 3.27 | 2.60 - 4.11 | 0.000 | |
| Household-level risk factorsa | |||||
| Number of households | |||||
| ≥ 7 | 236/352 (67.04) | 1 | |||
| 5 - 6 | 617/958 (64.40) | 0.90 | 0.67 - 1.21 | 0.501 | |
| ≤ 4 | 574/903 (63.56) | 1.03 | 0.75 - 1.42 | 0.828 | |
| Father education | |||||
| Illiterate | 163/260 (62.69) | 1 | |||
| Primary | 472/709 (66.57) | 1.28 | 0.89 - 1.84 | 0.181 | |
| Secondary | 379/595 (63.70) | 1.17 | 0.78 - 1.75 | 0.436 | |
| Tertiary | 391/613 (63.78) | 1.23 | 0.78 - 1.92 | 0.362 | |
| University | 141/241 (58.51) | 0.89 | 0.50 - 1.58 | 0.713 | |
| Mother education | |||||
| Illiterate | 304/459 (66.20) | 1 | |||
| Primary | 517/792 (65.27) | 1.08 | 0.79 - 1.48 | 0.614 | |
| Secondary | 338/530 (63.77) | 1.04 | 0.72 - 1.51 | 0.817 | |
| Tertiary | 324/526 (61.60) | 1.02 | 0.68 - 1.54 | 0.892 | |
| University | 79/139 (56.83) | 0.63 | 0.33 - 1.19 | 0.161 | |
| Father job | |||||
| Unemployed | 98/154 (63.63) | 1 | |||
| Hand-worker | 344/534 (64.41) | 1.09 | 0.70 - 1.71 | 0.680 | |
| Government-employee | 373/577 (64.64) | 1.20 | 0.73 - 1.97 | 0.464 | |
| Farmer | 183/275 (66.54) | 1.09 | 0.67 - 1.79 | 0.706 | |
| Self-employed | 530/849 (62.42) | 1.07 | 0.68 - 1.66 | 0.763 | |
| Mother job | |||||
| Homemaker | 1383/2182 (63.38) | 1 | |||
| Employed | 174/256 (67.96) | 1.73 | 1.14 - 2.62 | 0.009 | |
| HAV positive | HAV negative | ||||
| Socioeconomic status (score), mean | 7.21 | 7.24 | 0.97 | 0.92 - 1.04 | 0.494 |
| Province-level risk factors (mean) | |||||
| Healthy drinking water (%) | 93.09% | 93.08% | 1.01 | 0.98 - 1.02 | 0.659 |
| Budget (ratio) | 0.95 | 0.97 | 0.49 | 0.16 - 1.49 | 0.214 |
| Population density (N/m2) | 140.89 | 146.38 | 0.99 | 0.99 - 1.00 | 0.900 |
Household- and Province-Level Risk Factors for Hepatitis A Infection in 10 to 18-year-old Iranian Adolescents by Multilevel Analysis: the CASPIAN III Study
About 10% and 18% of fathers and mothers were illiterate, respectively; the percentages of university-educated parents were 10% and 5% for fathers and mothers, respectively. Risk for HAV infection was lower for students with university-educated parents than for those with illiterate parents (fathers: OR, 0.89; mothers: OR, 0.63); however, these differences were not statistically significant. The father’s job was not a significant risk factor for HAV prevalence. Although most mothers were homemakers (89.5%), HAV infection was significantly more prevalent in children whose mothers worked outside of the home (OR, 1.73; 95%CI, 1.14-2.62; P = 0.009).
The score for SES ranged from 4-11 (mean = 7.22) and was not related to HAV infection. Similarly, the province-level risk factors such as healthy drinking water (pipe or bottled water), population density, and the ratio of household income to costs were not associated with HAV prevalence.
5. Discussion
This large study showed that the majority (> 50%) of adolescents in all provinces of Iran have immunity to HAV. Rates of immunity in some provinces such as Fars (50.43%), Kordestan (52.80%), and Razavi Khorasan (52.83%) were much lower than in others. These results suggest that in these provinces, a transition from intermediate to low endemicity is occurring. In this situation, the main transmission route is from person-to-person and is often associated with a community-wide outbreak (2).
The current study revealed that despite some differences in HAV prevalence in the studied provinces, all areas of Iran have intermediate HAV endemicity. Therefore, according to the recommendation of the World Health Organization (2), childhood HAV vaccination is recommended in all provinces irrespective of socioeconomic level, especially in provinces that are transitioning from intermediate to low HAV endemicity.
Our findings are consistent with prior reports on HAV seroprevalence in several provinces of Iran. HAV seroprevalence was 86.76% in 17-year-old adolescents in the Golestan province (13), 61.6% in individuals under 20 who were referred to a hospital in Tehran (14), 79.3% in 15 to 20-year-old persons referred for pre-marriage consultations in the Fars province (9), 64.7% in 15 to 24-year-old adolescents in Mashhad in the Razavi Khorasan province (15), and 79.4% in 16 to 29-year-olds in Shiraz, the provincial city of the Fars province (8). In contrast, HAV seroprevalence was 38.9% in under 25-year-olds in Sari (center of Mazanderan province) (16), 10% in 6-20-year-olds in a population- based study in Isfahan province (17), and 3.9% in children under 15 in Kashan (in the Isfahan province) (18), which are much lower than in other reports.
The major difference between our study and other reports is the method of case selection and the sample size. In our study, the samples were selected by a multistage cluster method and adjusted by sex and age from the total inhabitants of each province, whereas other studies in Iran were mainly performed in a restricted area in a province and had recruited subpopulations, such as hospital referees or medical students.
Previously, we reported that HAV seropositivity was not related to gender or to residence area (urban/rural), and that seropositivity increased with age (3). This study investigated the household- and provincial-level risk factors for HAV. Provincial factors such as healthy drinking water, household income ratio to costs, and population densities were not related to HAV seropositivity. In addition, household factors such as household size, parents’ education, father’s job, and socioeconomic status were not associated with HAV seroprevalence. The only factor that was significantly related to HAV prevalence was the mother’s job. We found that children whose mothers worked outside of the home were more likely to be HAV positive than those with homemaking mothers. In Iran, most mothers are homemakers (about 90% in our study) and commonly take care of their children at home until the children are school aged. Frequent contact of children in day care centers probably increases the risk of acquiring HAV infection in children with working mothers. In this situation, the burden of symptomatic HAV infection in the HAV seronegative households of these children could be significant.
Several studies in Iran have assessed the risk factors for HAV infection; our results are in agreement with the study in the Golestan province, in which the number of family members, level of education of the person, and level of education in parents were not statistically different between the HAV positive and negative adolescents (13). The same results are reported from Kashan, Qum, and Isfahan, with no detectable association between HAV infection and family size (17-19). Another study in a district of Mazandaran found that educational levels, water supply, and sewage disposal systems did not affect HAV epidemiology (20). Conversely, in a sample of medical students in Tehran, clean water availability and higher levels of parents’ education were protective factors against the risk of HAV seropositivity (21), and in Fars province, higher household size was related to higher HAV prevalence (9). Sample size and sampling method might have influenced the results; however, it seems that basic infrastructures such as safe drinking water supply and sewage disposal, as well as primary health care education, which are the main determinants of HAV acquisition are to a large extent provided in most regions of the country (12).
Numerous studies on HAV seroprevalence and its risk factors in neighboring countries of Iran have been published. In Turkey, the seroprevalence in children under the age of 18 varied between 29.5% to 57% in different regions, and several parameters such as low socioeconomic status of family, low family income, large family, low education of the parents (especially mothers), and unsafe drinking water were frequently recognized as risk factors for HAV (22-25). In Kuwait, the seroprevalence was 24% in the age group of 18-27 years and was associated with non-educated parents (26). In Cairo, Egypt, HAV prevalence was much higher among children aged 3-18 with low and very low socioeconomic status (90%) compared to children with high socioeconomic status (50%) (27). In Jordan, approximately 70% of 10 to 18--year-old adolescents were seropositive for HAV, primarily those with lower maternal education, as well as those who lived in areas with unsafe drinking water and sewage disposal (28).
In other regions of the world with high or intermediate HAV endemicity, such as India with an overall prevalence of over 90% (29) and Brazil with a 58.8% seropositivity rate in adolescents (30), similar risk factors have been documented. For example, in India, personal hygiene (especially hand and food hygiene) was the main determinant, and in Brazil, living on crowded campuses and drinking well water were the major risk factors (29, 30). However, in regions with low endemicity such as Canada, with a rate of 2.7% seropositivity in 8-13-year-olds the main risk factor was being born in or having a history of travel to an endemic country (31).
Iran is located in the strategic region of the Middle East, where wars and resulting population displacements commonly cause infectious disease outbreaks, including HAV, not only in source countries but also in neighboring regions that host refugees (32). For instance, HAV outbreaks have recently been reported from Syrian refugee camps in Iraq, Jordan, and Lebanon (33). Thus, universal HAV vaccination seems to be a serious health issue in Middle Eastern countries.
Limitations: We were not able to cover all provinces of the country; however, the provinces we did include accounted for more than 70% of the total population of the country and are from different socioeconomic levels. Although basic sampling in the main study was proportional to sex, age, and rural/urban residency, the available samples from some provinces did not meet the true ratios, and weighting was used to compensate for this bias.
5.1. Conclusions
Although all studied provinces in Iran had intermediate endemicity for HAV infection and the risk of symptomatic infection in adults of all provinces was considerable, the risk in some provinces (such as Fars, Kordestan, and Razavi Khorasan) that are transitioning from intermediate to low endemicity is higher. Public education about HAV and preparation for outbreak control should be considered in these provinces. Indeed, universal HAV vaccination is recommended in all provinces of Iran irrespective of socioeconomic level. Children whose mothers work outside of the home are at higher risk of acquiring HAV infection at an early age and transmitting the infection to their family; therefore, there is a need to inform families of this risk.