1. Background
Hepatitis C virus (HCV) infection is a growing global health issue, with the individuals living with chronic HCV infection are at risk of developing advanced liver disease such as cirrhosis and hepatocellular carcinoma (HCC) (1). People who inject drugs (PWID) are currently the main population at risk for HCV infection in Iran, which is in contrast to the most other countries in the Middle East and Eastern Mediterranean region, where iatrogenic exposure drives HCV epidemics (2). Iran is categorised as a low-HCV prevalence country (3). However, HCV seems to emerge as the leading cause of viral hepatitis-related advanced liver disease and death in the near future in Iran given the high coverage of hepatitis B virus (HBV) vaccination in infants and implementation of HBV vaccination programs among adolescents (4-6).
Progression to advanced liver disease in individuals living with chronic HCV infection can be prevented by timely HCV treatments (7, 8). Interferon (IFN)-based treatments were previously the standard of care for chronic HCV infection and are widely used in Iran. Domestic Peg-IFN is also available in Iran with a much lower price than the imported Peg-IFN and a good efficacy with 78% of the patients achieving sustained virologic response (SVR) (9). However, IFN-based treatment is generally poorly tolerated, has a prolonged treatment course, and has a suboptimal efficacy which limits the treatment uptake and the impact of treatment on HCV disease burden in a population level.
The development of IFN-free direct-acting antiviral (DAA) therapies for HCV infection in the recent years has resulted in a realistic optimism to increase treatment uptake and decreasing the burden of HCV infection given the high efficacy, broad eligibility criteria, limited side effects, and short duration of the new treatments (10, 11).
Although utilisation of IFN-free DAA therapies has potentials to improve the public health management of HCV infection in Iran, data of HCV disease burden is crucial to guide public health strategies in providing the best levels and settings of access to the new treatments. Although there are strong data of epidemiology of HCV infection in the Iranian general population (12-17) and in the populations at a greater risk of HCV infection (18-21), there are limited data estimating HCV-related mortality and morbidity in Iran.
2. Objectives
This study was conducted to characterise the current and future liver disease burden of chronic HCV infection in Iran using the available data and to assess the impact of various treatment and diagnosis strategies on the projected HCV-related liver disease burden.
3. Materials and Methods
A mathematical model of HCV transmission and progression was used. The flow of the HCV disease progression model was illustrated in the supplementary diagram (Appendix 1), with a detailed description of the model and associated parameter estimates being previously reported (22). In summary, the model was constructed in Microsoft Excel (Microsoft corporation, Redmond, WA, USA) to estimate the number of individuals with chronic HCV infection (total viremic, diagnosed and treated), the number of individuals with various HCV-related hepatic fibrosis stages, including cirrhosis, and HCV-related mortality. All the estimates reported in the text were rounded to the nearest 100 for the number of HCV infections and to the nearest 10 for advanced liver disease and mortality numbers. The data of historical epidemiology of HCV infection in Iran was collected through a literature search, and discussion with an expert panel. Iran-specific inputs and assumptions used in the model are summarised in Table 1, while a more detailed description comes as follow.
| Input Variable | Value | Year (Reference) |
|---|---|---|
| Total population | 78,470,000 | 2014 (23) |
| HCV prevalence (HCV antibody) in adult population | 0.50% | 2006 (13) |
| HCV prevalence (HCV antibody) in general population | 0.39% (0.27% - 0.51%)a | 2006 (extrapolated from (13)) |
| Viremic proportion out of HCV antibody positive individuals | 62% | 2008 (14) |
| Viremic prevalence | 0.24% (0.17% - 0.31%)a | 2006 (calculated based upon (13, 14)) |
| HCV diagnosis | 35% | 2014 [expert consensus] |
| HCV incidence (annual) | 11 per 100,000 | 2014 [estimated in the current study] |
| HCV treatment rate (annual) | 2.4% | 2014 (calculated based upon expert consensus, PegIFN units sold, and (24)) |
| Infected via IDU | 75% | 2007 (19, 25) |
| Genotype distribution | Genotype 1: 64%; Genotype 2: 2%; Genotype 3: 33%; Genotype 4: 1% | 2003 - 05 (24) |
Model Inputs and Assumptions
3.1. Total Population
The data of the population size in Iran were obtained from the United Nations world population prospects (esa.un.org/Wpp) (23), which uses the data of the Iranian Population and Housing Censuses implemented by statistical center of Iran (http://amar.org.ir).
3.2. HCV Prevalence and Genotype Distribution
Age and gender- specific HCV antibody prevalence estimates were based upon a household survey investigating 5,684 individuals, > 18 years old, from three provinces in 2006 (13). HCV prevalence in population < 18 years was estimated, trending an exponential decline from age 18 to 0.
Viremic population or individuals living with chronic HCV infection is defined as individuals with an HCV RNA positive. Viremic proportion among HCV antibody positive individuals in general population was based upon a large population-based survey investigating 49,338 individuals (14).
HCV genotype distribution was based upon a study of 2,231 HCV positive samples from a reference laboratory (24).
3.3. HCV Incidence
Data of HCV incidence was not available and it was therefore back-calculated. At any time point, the total number of HCV infections equals the sum of all new infections minus the number of spontaneously cleared, deceased and cured cases, as presented below:
Equation 1.
The annual number of new cases was calculated using the known number of total HCV infections in 2006 (13). The annual number of all-cause mortality, liver-related deaths and cured cases was estimated by the model, as previously described (22). The incidence was set to one in 1950 and an expert consensus was used to define the historical changes in HCV incidence and to identify the years when HCV incidence peaked considering the most common risk factors in the previous years. The age and gender distribution of the acute infections were calculated using the age and gender distribution of the total infected population in 2006 (13).
3.4. HCV diagnosed Population
Estimation of HCV diagnosis rate was based on an expert consensus. It was assumed that 35% of total individuals living with HCV infection in 2014 had been diagnosed, including 6,000 newly diagnosed and 60,000 diagnosed in the previous years. An annual 6,000 newly diagnosed individuals were assumed, beginning from 2014.
3.5. HCV Treated Population
Estimation of annual HCV treatment rate was based on an expert consensus and available data, using approximate number of Peg-IFN units sold in Iran in 2014, HCV genotype distribution (24), treatment duration in each genotype, and the percentage of individuals completing treatment (80%, expert consensus).
3.6. Liver Disease Progression and Mortality
Age and sex specific transition probabilities were modelled to progress HCV infected individuals annually through each liver fibrosis/disease stage. Age and sex specific mortality rates were estimated based upon Human Mortality Database of the University of Berkeley (26), adjusted for excess mortality due to injection drug use. More details were described in an earlier work (22).
3.7. HCV Diagnosis and Treatment Strategies
One base case scenario and five alternative scenarios were developed, assuming various levels of diagnosis and treatment rate to assess the impact of various strategies on the future HCV disease burden in Iran by 2030. Among the five alternative scenarios, the first four scenarios assumed various levels of treatment rate, while in the fifth scenario a target for HCV infection reduction was assumed and the diagnosis rate required in addition to the treatment rate assumed in the previous scenario to achieve the target was assessed. The assumptions used in all scenarios have been summarised in Table 2.
| Scenario | Treatment Regimen | Newly Diagnosed Ratea | Treatment Ratea |
|---|---|---|---|
| Base case | IFN-based | 6,000 | 4,500 |
| Scenario 1: Increasing treatment efficacy only | IFN-free DAA | 6,000 | 4,500 |
| Scenario 2: Treating advanced liver fibrosis only | IFN-free DAA | 6,000 | Only liver fibrosis stage ≥ F3 |
| Scenario 3: Doubling treatment rate | IFN-free DAA | 6,000 | 9,000 (2016 - 2030) |
| Scenario 4: Stepwise increase in treatment rate | IFN-free DAA | 6,000 | 9,000 (2016 - 2017); 18,000 (2018 - 2030) |
| Scenario 5: Targeting at least 90% reduction in total HCV infected individuals by 2030 | IFN-free DAA | 6,000 (2016 - 2017); 12,000 (2018 - 2019); 24,000 (2020 - 2030) | 9,000 (2016 - 2017); 18,000 (2018-2030) |
Treatment Regimens, Diagnosis Rates and Treatment Rates Assumed in Each Scenario
The base case scenario assumed the current utilisation of IFN-based treatment with the average SVR of 58% (genotype 1), 70% (genotype 2), 78% (genotype 3), and 61% (genotype 4). It was assumed that individuals aged 20-64 years are considered for treatment, including those with liver fibrosis ≥ F0 for genotype 3 and ≥ F1 for all other genotypes, while 60% of the patients are eligible for IFN-based treatment, based on clinical guideline recommendations for IFN contraindications and expert consensus on routine clinical practice in Iran. As the most conservative strategy, this scenario assumed that the annual number of newly diagnosed individuals and those receiving treatment remained the same as those estimated for 2014.
Scenario 1 assumed the utilisation of IFN-free DAA treatments with the SVR of 90% (genotype 1), 85% (genotype 2), 78% (genotype 3), and 58% (genotype 4) in 2015, increasing to 95% (all genotypes) in 2016. It was assumed that all liver fibrosis stages are considered for treatment, while 90% of the patients are eligible for treatment. Annual numbers of newly diagnosed and treated individuals were held constant to those estimated for 2014.
Scenarios 2 to 5 assumed the utilisation of IFN-free DAA treatment with the same SVR similar to those in scenario 1 but different assumptions in treatment eligibility, diagnosis rate and treatment uptake were implied, as described below.
Scenario 2 assumed restricting treatment eligibility to individuals with a liver fibrosis stage ≥ F3. Scenario 3 assumed no liver fibrosis-based treatment restriction in addition to doubling the number of treated individuals in 2016 (9,000 annually), as compared with scenario 1. Scenario 4 assumed a stepwise increase in treatment uptake. It is assumed that 9,000 individuals received treatment annually from 2016 to 2017 (doubled), while this number increased to 18,000 individuals annually from 2018 onward (quadrupled). Scenario 5 targeted at least 90% reduction in the total number of individuals living with HCV infection by 2030. To achieve this target, the same increase in treatment uptake as that in scenario 4 was assumed in addition to sufficient increases in diagnosis rate. This scenario assumed the annual number of individuals newly diagnosed with HCV infection increased from 6,000 (2016 to 2017) to 12,000 (2018 to 2019), and 24,000 (2020 onward).
4. Results
4.1. Base Case Scenario
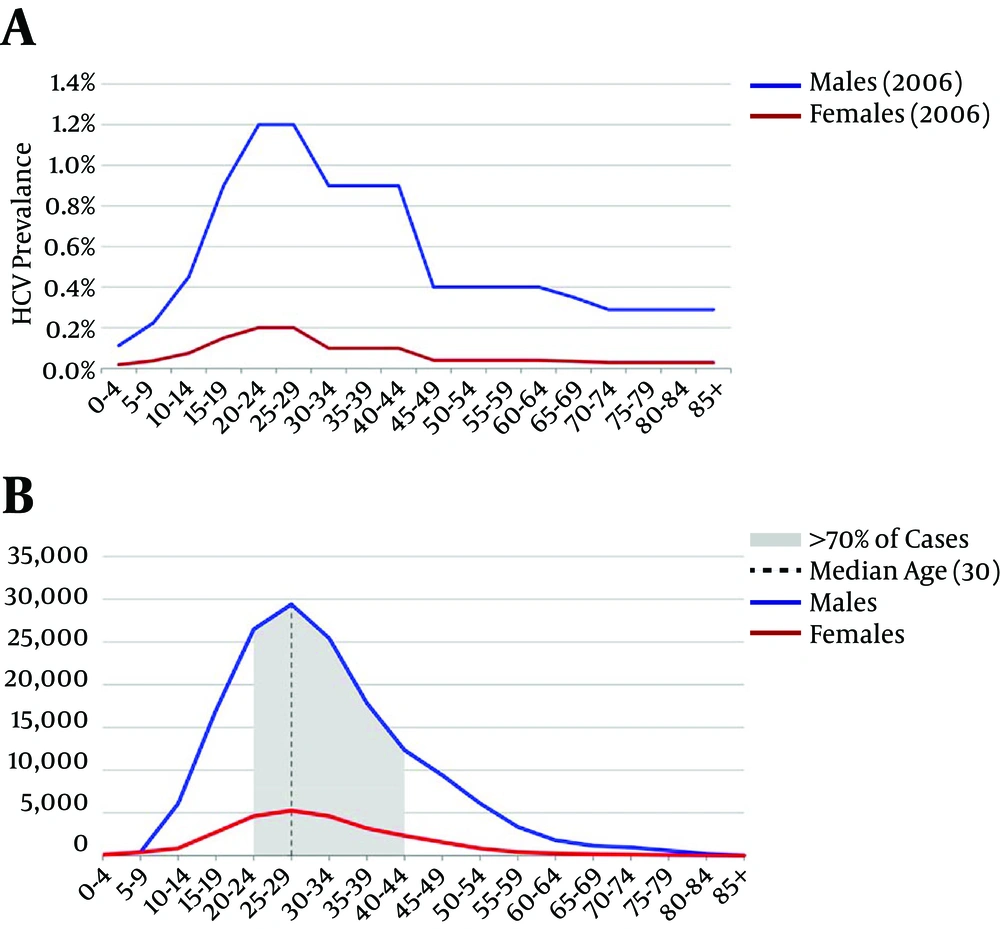
It is estimated that 186,500 individuals were living with chronic HCV infection in Iran in 2014, with a median age of 30 years and those between 20 and 44 years old constituted 70% of total HCV infected population (Figure 1B). Estimated number of individuals with HCV-related decompensated cirrhosis (DC), HCC and death in 2014 were 140, 160 and 120, respectively.
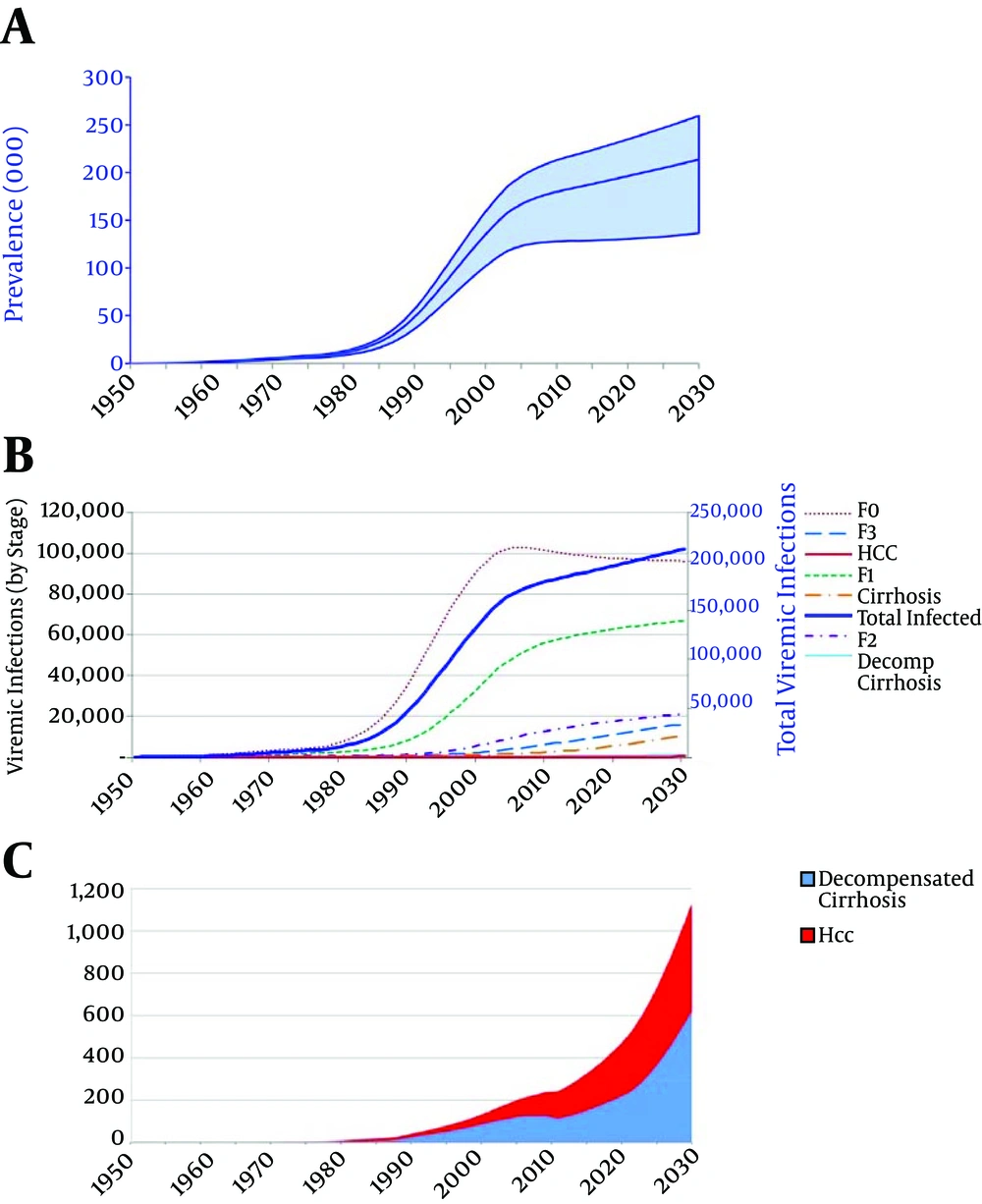
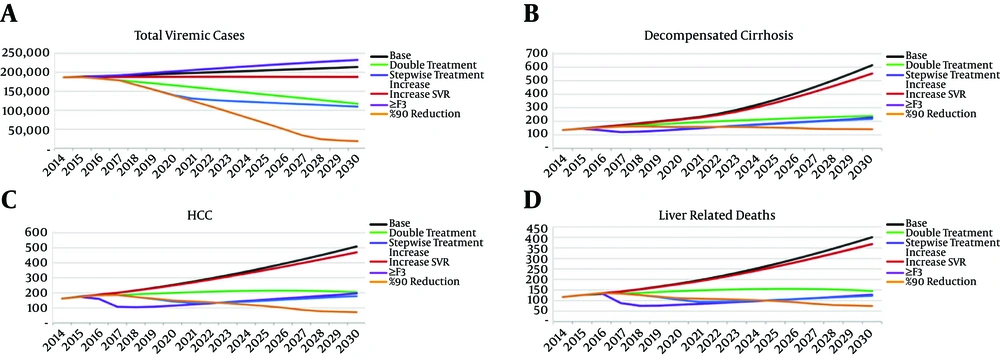
Under the base case scenario, assuming utilisation of IFN-based treatment and the current diagnosis and treatment rates, an upward trend in number of HCV infections and an exponential increase in advanced liver disease is expected during the next decades (Figure 2). By 2030, the estimated number of individuals living with chronic HCV infection is expected to increase to 213,700. Moreover, the number of individuals with DC, HCC and liver disease death is expected to increase 4.4 fold (n = 620), 3.2 fold (n = 510), and 3.3 fold (n = 400) by 2030, respectively. The impact of base case scenario and the other five alternative scenarios on projected HCV disease burden by 2030 has been illustrated in Figures 3 and 4.
A, HCV antibody positive prevalence in general population in 2006 (extrapolated from (13); B, estimated number of individuals living with chronic HCV infection in 2014. Grey area represents the 70% of the total HCV infected population. Vertical line represents the median.
A, Total number of individuals living with HCV infection. Shaded area represents 95% confidence interval; B, number of individuals living with HCV infection in total and by liver fibrosis and disease stage; C, number of individuals with HCV-related decompensated cirrhosis and hepatocellular carcinoma.
4.2. Scenario 1: Increasing Treatment Efficacy Only
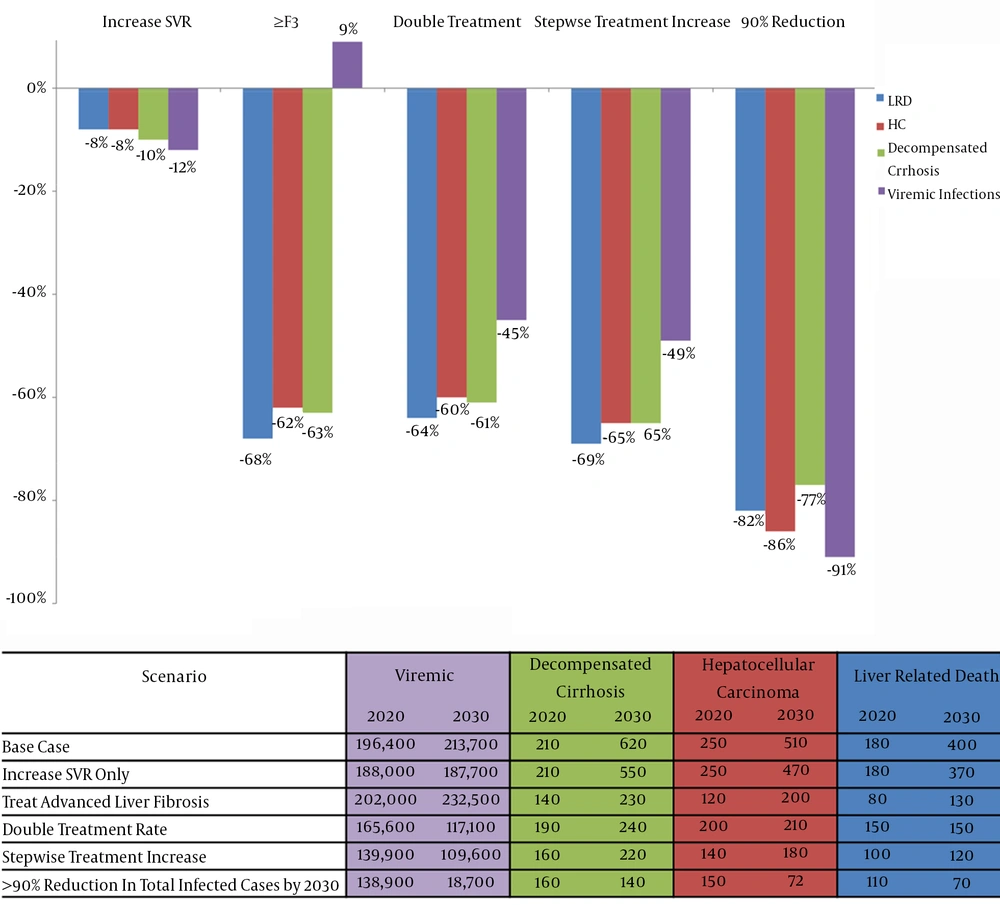
Scenario 1 assumed replacing IFN-based treatments with IFN-free treatments with no change in treatment and diagnosis rate. Under this scenario, a still increasing trend is expected in HCV morbidity and mortality during the next decades (Figure 3). As compared with the base case scenario, this strategy has limited impact on HCV disease burden, with only 12% reduction in total number of HCV infected individuals and 8% - 10% reduction in the numbers of advanced liver disease and death cases being expected in 2030 (Figure 4).
4.3. Scenario 2: Liver Fibrosis-Based Treatment Restriction
Scenario 2 assumed restricting HCV treatment to individuals with severe liver fibrosis (stage ≥ F3). Under this scenario, the number of individuals with DC, HCC and liver-related death will be decreased by 63% (390 cases averted), 62% (310 cases averted) and 68% (270 deaths averted) by 2030, respectively, as compared with the base case scenario. However, this strategy does not control the number of HCV infected population, resulting in a 9% increase (18,800 cases added) in the total number of individuals living with HCV infection by 2030 as compared with the base case scenario (Figure 4).
4.4. Scenarios 3, 4, and 5: Increasing Diagnosis and/or Treatment Rates
Scenario 3 assumed a modest increase in treatment uptake to two times higher than the current level (9,000 patients annually). The model showed that even this modest increase in treatment uptake will have a major impact on HCV epidemic, resulting in a downward trend in the number of individuals living with HCV infection during the next decades (Figure 3). As compared to the base case scenario, this strategy will also result in 61% reduction in the number of DC (380 cases averted), 60% reduction in the number of HCC (300 cases averted) and 64% reduction in mortality (250 deaths averted) by 2030 (Figure 4).
Scenario 4 assumed a more aggressive but stepwise increase in treatment uptake with no change in diagnosis rate. Although substantial reduction in HCV morbidity and mortality will be achieved under this strategy as compared with the base case scenario, this strategy is limited in further decreasing advanced liver disease, and mortality after 2021 - 22 due to the low diagnosis rate (Figure 3). It means that the underdiagnosed pool of HCV infected population will drive HCV disease burden in spite of increasing treatment uptake.
Scenario 5 targeted at least 90% reduction in the total number of individuals living with HCV infection by 2030. Marked increases in both treatment uptake and diagnosis rate, as described earlier, is required which will result in a substantial and steady reduction in HCV disease burden (Figure 3). As compared with the base case scenario, this strategy will result in 91% reduction in total number of HCV infected individuals (195,000 cases averted), 77% reduction in the number of DC (480 cases averted), 86% reduction in the number of HCC (438 cases averted) and 82% reduction in mortality (330 deaths averted) by 2030 (Figure 4).
5. Discussion
This study is the first to provide an estimation of the current and future HCV infection morbidity and mortality in Iran. The findings of this study indicated that under the current treatment and diagnosis rates and using IFN-based treatment, a marked and steady increase in number of HCV infections, and HCV-related advanced liver disease and mortality is expected in the next decades in Iran. It was demonstrated that a combination of enhanced treatment efficacy through utilisation of IFN-free DAA therapies, and increased diagnosis and treatment uptake is required to reduce the HCV disease burden in Iran.
The increasing trend in HCV morbidity and mortality, projected in this study, is mainly due to the aging of the currently young cohort of Iranian individuals living with HCV infection and the time lag of progression of chronic hepatitis to advanced liver disease. The young population with HCV infection in Iran provides an opportunity for timely interventions with antiviral therapy to prevent liver fibrosis progression and to avert the projected rising trend in HCV-related liver disease burden.
This current study indicated that utilisation of IFN-free DAA treatment as a single strategy or restricting treatment to individuals with severe liver fibrosis has limited impact on HCV morbidity and mortality while treatment scale-up with no fibrosis-based treatment restriction is needed to avert the impending rising of HCV disease burden in Iran. Although replacing the toxic and arduous IFN-based treatment with safe and short IFN-free DAA regimens is an important enabler factor to increase willingness to treatment (27), there are still other remaining barriers to HCV care (28-30) which should be addressed properly to ensure a successful treatment scale-up. Integration of substance use care and HCV care through provision of HCV care in opioid substitution therapy (OST) clinics and using peer-support services to engage PWID in HCV care were successful strategies in increasing adherence to treatment (31, 32). The feasibility of treatment scale-up strategies also depends on the capacity of the health system in serving new patients. Replacing IFN-based treatments by IFN-free DAA treatments per se has a potential to boost the current capacity of treatment services, given treatment duration will be shortened and on-treatment viral load and safety monitoring will be removed. However, some other strategies can be implemented to further facilitate access to treatment such as authorisation of trained general practitioners to prescribe HCV anti-viral agents in the remote areas or in the settings where access to specialist services is difficult or not cost-effective.
Our findings also indicated that a marked increase in number of individuals diagnosed with HCV infection is crucial in addition to increasing treatment uptake to achieve at least 90% reduction in the number of individuals living with HCV and substantially reduce the HCV disease burden. Two approaches in HCV screening can be suggested to increase diagnosis rate, including risk-based screening and mass screening. Risk-based screening programs target individuals at risk of HCV infection and can be implemented in the substance use services like OST clinics, prisons, and the centers providing care for individuals with HIV. Extensive efforts in both provincial and national levels are needed to widely implement HCV screening in the relevant settings. Mass screening approach with no pre-assessment of HCV risk behavior is an alternative strategy which removes any possible stigma attributed to injecting drug use. A birth cohort mass screening strategy was implemented in the United States for the individuals born during 1945 - 65 given the high risk of HCV transmission through blood transfusion during 1980s in this country (33). Given that men in young age represent the highest proportion of HCV epidemic in Iran (Figure 1), HCV screening during the mandatory military services may be an option for the mass HCV screening in Iran. A cost-effectiveness assessment is needed given that any mass screening program is costly and logistically difficult.
Although increasing diagnosis and treatment uptake is important in reducing HCV disease burden, harm reduction interventions should be also considered as an important factor affecting transmission. Modelling studies demonstrated that increasing coverage of OST and needle and syringe programs can substantially reduce the treatment rate required to achieve specific HCV prevalence reductions (34).
The number of individuals living with HCV infection and those with HCV-related HCC in Iran, estimated in the current study are different with those estimated in the latest Global Burden of Disease study (35). This is mainly because of the more details considered in the model used in the current study, including using viremic prevalence (HCV RNA positive) instead of HCV antibody positive prevalence.
This study has several limitations. Although the best available data were used to inform the model parameters, there was limited data of some parameters such as HCV incidence, number of individuals diagnosed with HCV infection and HCV treatment uptake. Community based surveys, including longitudinal studies of PWID and linkage of administrative data of HCV notifications and HCV drug dispensing are needed to provide more accurate data for the future modelling studies. Clinical trials SVR data was used in the model. SVR data of the real world treatment experiences and in Iranian centers could be different than clinical trial data. The current model also did not incorporate the residual risk of liver fibrosis progression and HCC after HCV cure (7). Therefore, the model could minimally overestimate the impact of SVR on overall HCV liver-related morbidity and mortality.
In summary, the current study indicated that utilisation of highly effective IFN-free DAA therapies should be coupled with dramatic increases in treatment uptake and diagnosis rate, along with ongoing prevention interventions (such as OST and needle and syringe exchange services) to control HCV morbidity and mortality in Iran. Recently, a domestic biosimilar IFN-free DAA (Sovodak) has been introduced with a high efficacy and a good safety profile which is covered by the health insurance system, providing a good opportunity for scaling-up HCV treatment in Iran. Given the relatively young age of the HCV infected population in Iran, HCV treatment scale-up is a timely intervention to reverse the rising tide of HCV burden in the future. Further studies are needed to assess the cost-effectiveness of each strategy suggested in this study and evaluate the capacity of the health system in implementation of HCV diagnosis and treatment scale-up.