1. Context
Hepatitis B virus (HBV) infection is a major infectious disease with worldwide prevalence (1, 2). 1 - 2 billion people are estimated to be afflicted with HBV (2, 3), and 0.4% of healthcare workers (HCW) are suffering from the disease (4). In addition to reducing the level of exposure and taking protective measures during diagnostic and therapeutic procedures, vaccination currently is the most effective way to prevent HCWs from HBV (5, 6). Iran has been conducting the vaccination program for high-risk groups, such as medical staff and clinical students, at intervals of 0, 1, and 6 months since 1993 (7). Systematic reviews and meta-analysis studies have reported the rate of HBV vaccination coverage to be 70.1%, 73%, and 72.2% for physicians, nurses, and dentists in Iran, respectively (8-10). Since the contact with infected blood is a possible route of disease transmission, HCW is at high risk of HBV (11). Anti-HBS titer is used to evaluate the efficacy of HBV vaccine and the levels of anti-HBs higher than 10 mIU/mL are considered as a positive response (12). Several studies conducted to evaluate the efficacy of HBV vaccine among HCWs in Iran have estimated the efficacy of the vaccine to be 67% - 100% (13-29). The most important factors affecting the efficacy of HBV vaccine include: age, gender, genetics, smoking, vaccine type, vaccination dose, injection site, and the period after the last injection of vaccine (30-34).
2. Objectives
Meta-analysis is a process, in which data of various researches, which share common ground, are collected and analyzed to get an authentic estimation of the effects of some medical interventions (35). It is quite clear that, considering the amalgamation of the data of several separate researches, meta-analysis methods occupy larger sample sizes, less chances and possibilities, and increased significance of statistical findings. Specific conditions necessary for the implementation of meta-analysis make it a very reliable procedure. Given the mixed results of HBV vaccine efficacy among HCWs, we intend to examine all related reports and finally present a rough estimate of the general condition through a meta-analysis study (36, 37).
3. Data Sources
The present study was performed according to the PRISMA guidelines for systematic review and meta-analysis studies (37). To avoid bias, two researchers independently was performed the all steps of meta-analysis including database searching, study selection, quality assessment, and data extraction. A comprehensive search was conducted based on national and international database: Magiran, Iranmedex, IranDoc, SID, Medlib, Scopus, Pubmed, Science Direct, Cochrane, Embase, Web of Science, Springer, wiley online library, Trials Register, DOAJ, and Google Scholar search engine without time limit up to 2016. Persian and English keywords compatible with MeSH terminology were used; these keywords included “Hepatitis B”, “Antibody”, “Vaccination”, “Vaccine”, “Immunization”, “ Immunogenicity”, “Efficacy”, “Healthcare", “Health Personnel”, “Students”, “Iran” and combined words using AND/OR Boolean operator. The combined search for PubMed is shown in Appendix 1.
3.1. Definitions
HBV vaccination response was considered positive when the antibody level was more than 10 (Anti-HBS > 10 mIu/mL). Healthcare workers included both healthcare students such as any students under education in hospitals and healthcare personnel such as any staff employed in hospitals, health centers affiliated in Iran in this study. (12).
3.2. Inclusion and Exclusion Criteria
Inclusion criteria included all HCWs with a history of HBV vaccination, and the time interval of 1-6 months since the last vaccination.
Exclusion criteria were lack of full vaccination at the time intervals of 0, 1, and 6 months, smoking, taking immunosuppressive drugs, uncertainty in regard to the time of the last vaccination, Anti-HBC (+) and HBs Ag (+), cases with booster dose of HBV vaccine, irrelevant studies, and insufficient data.
3.3. Quality Assessment
The researchers examined the quality of the selected studies in the next step using STROBE (38) as a standard checklist. This checklist included 22 items covering all aspects of the methodology of studies, such as sampling techniques, measured parameters, statistical analyses, and objectives. Each item was scored from 0 to 2 in the checklist and maximum possible score was 44. Consequently, the studies were divided into three categories: low quality with a score below 15.5, moderate quality with a score of 15.5 - 29.5, and high quality with a score of 30 - 40; those studies which gained the minimum score (15.5) were selected for the meta-analysis study.
4. Data Extraction
All papers which finally entered the process of this study were extracted according to a pre-prepared checklist. The checklist collected the following information: author’s name, year of study, place of study, type of study, sample size, medical personnel sample size, clinical students sample size, age and vaccine efficacy, for male and female separately.
4.1. Statistical Analysis
Variance of each study was calculated according to binomial distribution. Cochran test and I2 index were used to assess the heterogeneity of the studies. The heterogeneity in the present study was 88.8% indicating a high heterogeneity (I2 index less than 25% means low heterogeneity, 25% to 75% moderate heterogeneity, and more than 75% high heterogeneity) (39, 40). Regarding the significant heterogeneity index (I22), random effects model was used to pooled analysis in this meta-analysis. The studies were mixed together based on variance and sample size. Meta-regression model was used to find the relationships between vaccine efficacy and the year and sample size of the study. Subgroup analysis was done according to region and risk groups. STATA software Ver.11.1 was used to analyze the data. Egger test was performed to check publication bias. Egger test can indicate a symmetrical or asymmetrical funnel plot. P < 0.05 was considered as the significance level of the study.
5. Results
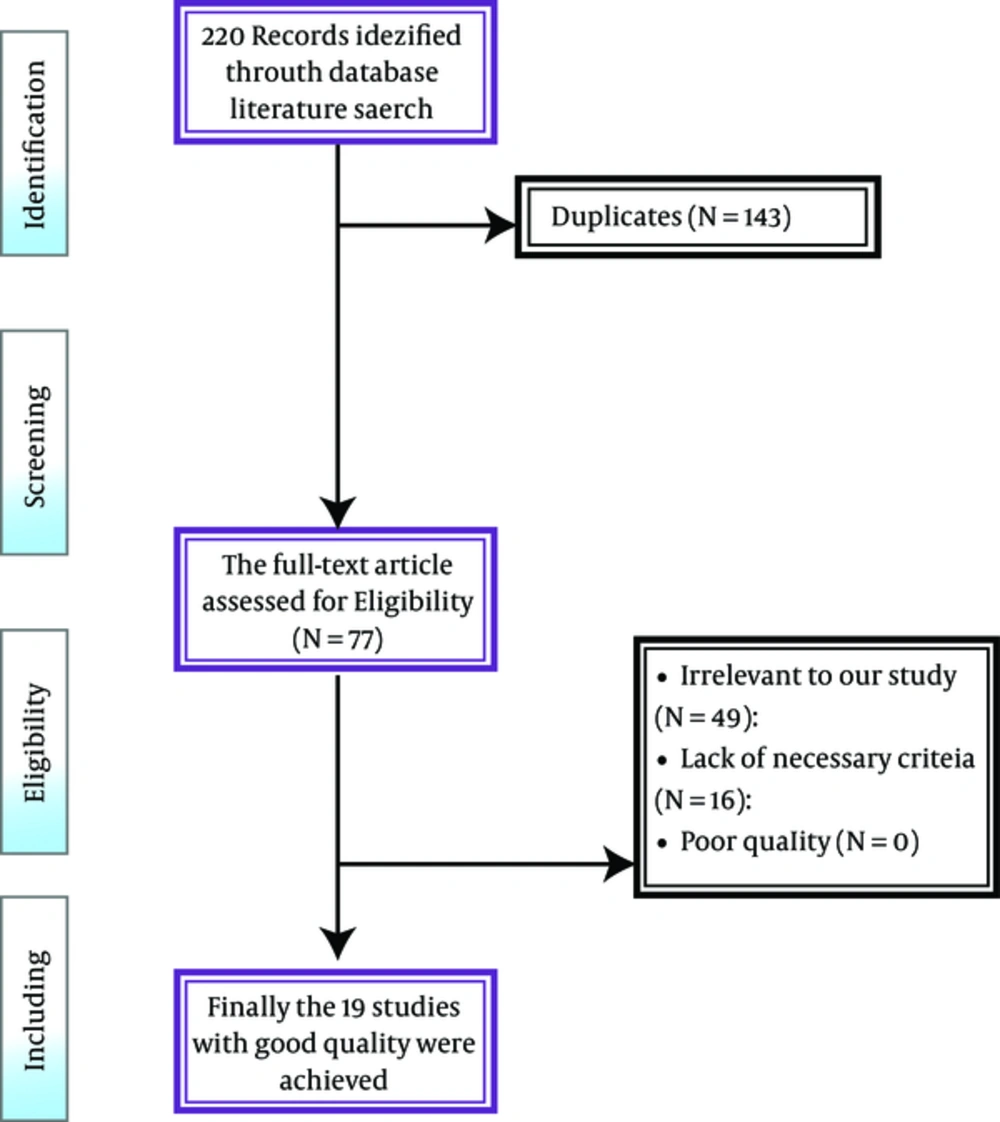
220 papers, after removing another 160 due to being repetitive, were identified in the systematic review. After analyzing the full text of 82 papers, 70 papers were eliminated for not meeting the inclusion criteria. Finally, 12 papers comprising 1726 healthcare workers (1388 healthcare personnel and 338 healthcare students) published during 1998-2015 were qualified to enter the meta-analysis study. Mean age of medical staff was 31.98 (confidence interval of 95%: 28.25 - 38.69) while the age range of clinical students was 20 - 26 years (Figure 1).
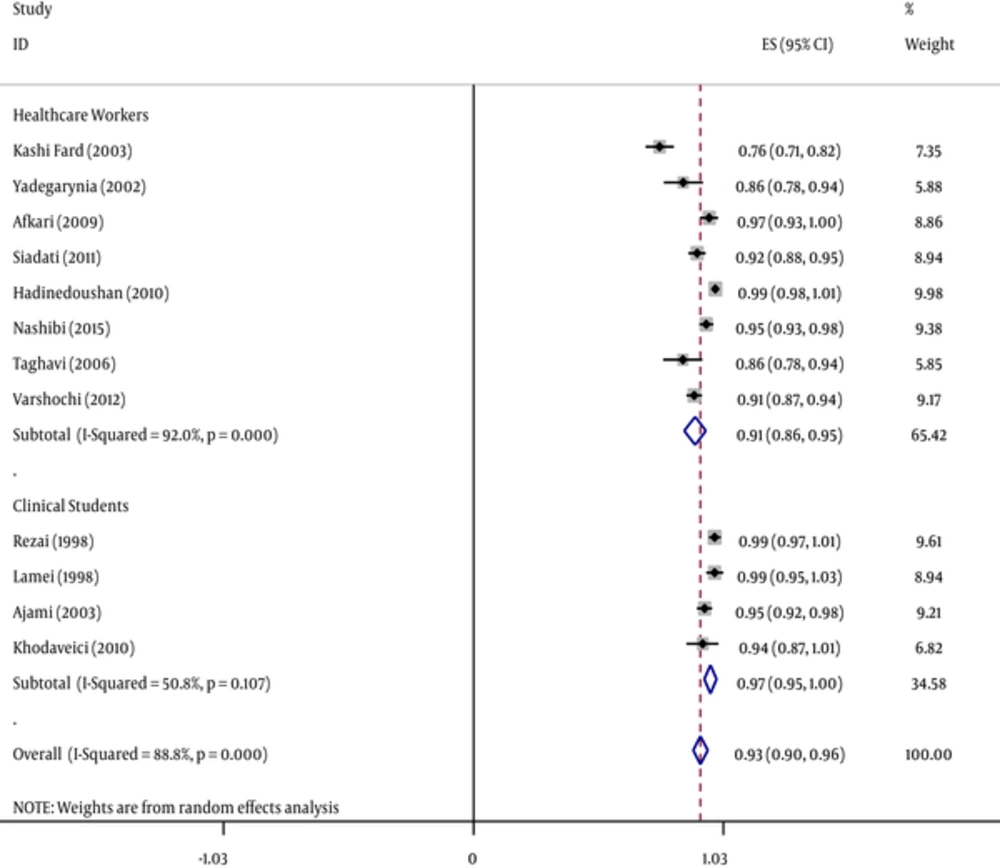
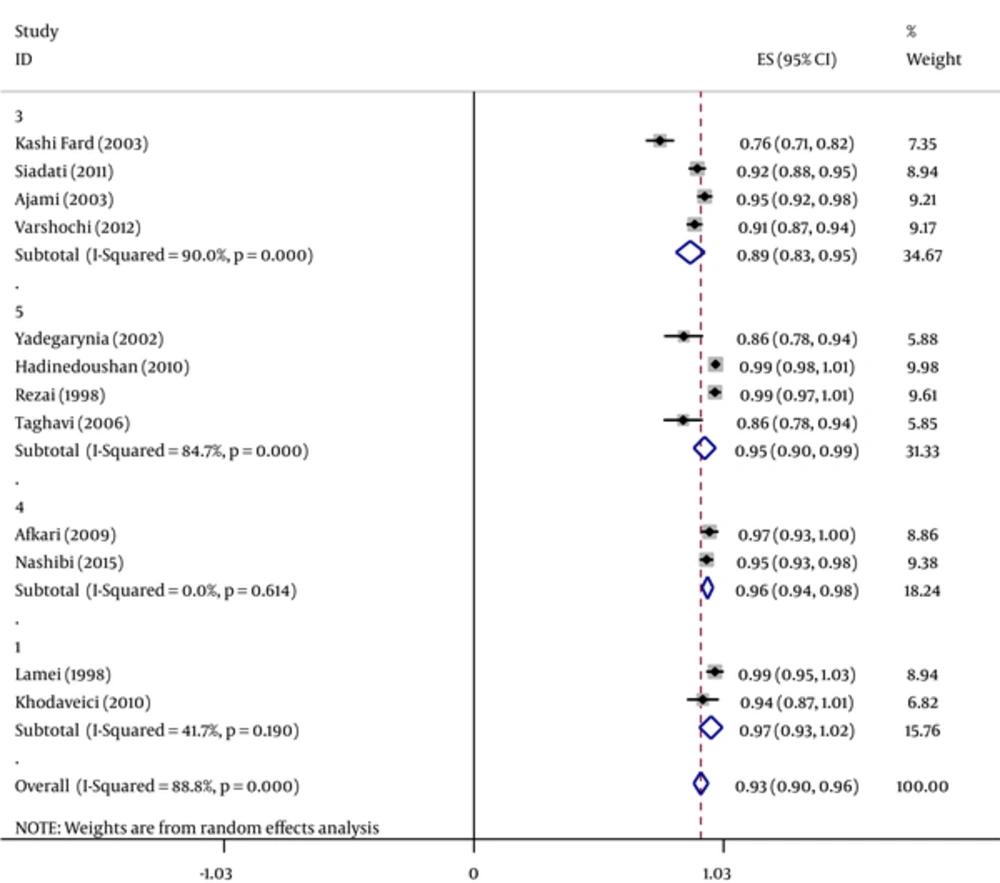
The efficacy of HBV vaccine, 1 - 6 months after the last dose of hepatitis B vaccination was estimated to be 93.1% (95% CI [Confidence Interval]: 90.3% - 97%) among HCWs. The lowest (76.4%) and highest (100%) rates were related to studies in Babol (2003) and Isfahan (1998), respectively. The efficacy of HBV vaccine based on geographical region is presented in Figure 2, which shows that the western part of Iran the most positive response as 97% (Tables 1 - 3).
Forest Plot of the Hepatitis B Vaccine Efficacy Separately Healthcare Students and Healthcare Personnel According to Anti-HBS Criteria Obtained by Random-Effects Model; the Midpoint of Each Line Segment Shows the Percentage Values; the Length of Line segments Shows 95% Confidence Interval in Each Study; Diamond Mark Shows the Efficacy of the Vaccine for all Studies
| Author Name | Location | Year | Sample Size | Risk Group | Average Age, y | Time Elapsed Since the Last Doses, mo | Vaccine | Doses of Vaccine, µg | The Injection Method | Vaccine Efficacy (%) AntiHBS > 10 mIU/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| Kashifard | Babol | 2003 | 240 | medical staff | 37 | 3 | HeperBiovax | 20 | intramuscular | 76.4 |
| Yadegarinia | Tehran | 2002 | 72 | medical staff | 3 | Heber Biovac | 20 | intramuscular | 86.1 | |
| Afkari | Lar | 2009 | 90 | medical staff | 38.5 | 3 - 4 | intramuscular | 96.7 | ||
| siyadat | Babol | 2011 | 230 | medical staff | 30 | 3 | Recombinant | intramuscular | 91.7 | |
| HadiNadooshan | Yazd | 2010 | 126 | medical staff | 22.4 | 4 | Recombinant | 100 | intramuscular | 99.2 |
| Rezaei | Esfahan | 1999 | 65 | Student | 1 - 2 | Recombinant | 20 | intramuscular | 100 | |
| lamei | Urmia | 1998 | 30 | Student | 34 | 1 | 100 | |||
| Ajami | Sari | 2003 | 193 | Student | 20 - 24 | 1 - 2 | Recombinant | 94.8 | ||
| Nashibi | Ahvaz | 2015 | 239 | medical staff | 1 - 6 | 95.6 | ||||
| Khodaveisi | Hamadan | 2010 | 50 | Student | 19 - 29 | 4 | Recombinant | intramuscular | 94 | |
| Taghavi | Tehran | 2006 | 72 | medical staff | 1 | Recombinant | 20 | intramuscular | 85.9 | |
| Varshuchi | Tabriz | 2012 | 319 | medical staff | 32 | 3 | EUVAXB | 90.6 |
| Risk Group | The Number of Studies | Sample Size | I2 | Confidence Interval (95%) | Total Estimated, % |
|---|---|---|---|---|---|
| Healthcare personnel | 8 | 1317 | 92 | 86.5 - 95 | 90.9 |
| Healthcare students | 4 | 338 | 80.8 | 95 - 97.7 | 97.2 |
| Gender | The Number of Studies | Sample Size | I2 | Confidence Interval (95%) | Total Estimated, % |
|---|---|---|---|---|---|
| Male | 8 | 380 | 86.7 | 93.1 - 98.6 | 95.9 |
| Female | 8 | 904 | 84.2 | 87.1 - 95.5 | 91.3 |
HBV vaccine efficacy was 90.9% (95% CI: 86.5 - 95.3) for 8 studies conducted on medical staff and 97.3% (95% CI: 94.7 - 97.7) for 3 studies on clinical students.
In regard to the immune response of HBV vaccine in health care staff and medical personnel, the rate was 95.9% (95% CI: 93.1 - 98.6) in males and 91.3% (95% CI: 87.1 - 95.5) in females (Figures 3 and 4).
Forest Plot of the Hepatitis B Vaccine Efficacy in Iranian Healthcare workers Separately in Different Geographical Areas According to Anti-HBS Criteria Obtained by Random-Effects Model; the Midpoint of Each Line Segment Shows the Percentage Values; the Length of Line Segments Shows 95% Confidence Interval in Each Study; Diamond Mark Shows the Efficacy of the Vaccine for all Studies (1 = West, 2 = East, 3 = North, 4 = South 5 = Center).
Table 4 shows the efficacy of HBV vaccine separately in clinical students and medical staff based on gender; the highest rate (99.6%) was related to male clinical students.
| Genus | Group | The Number of Studies | Sample Size | I2 | Confidence Interval | Total Estimated, % |
|---|---|---|---|---|---|---|
| Male | Medical Staff | 6 | 341 | 90.3 | 90.3 - 97.7 | 94 |
| Clinical students | 2 | 39 | 0 | 97.8 - 100 | 99.6 | |
| Female | Medical Staff | 6 | 700 | 88.7 | 84.8 - 96.2 | 90.5 |
| Clinical students | 2 | 204 | 0 | 90.3 - 97 | 93.7 |
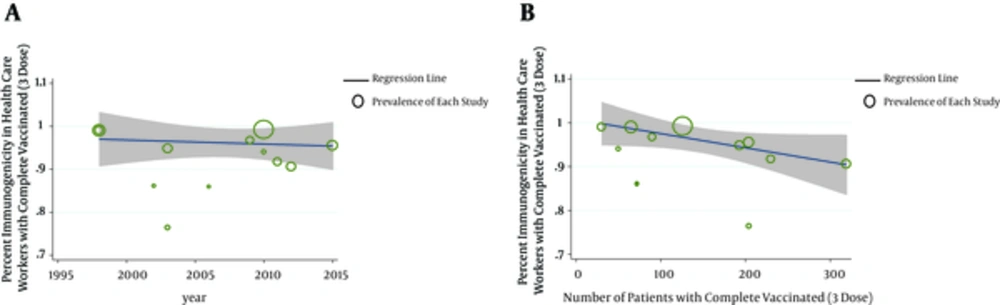
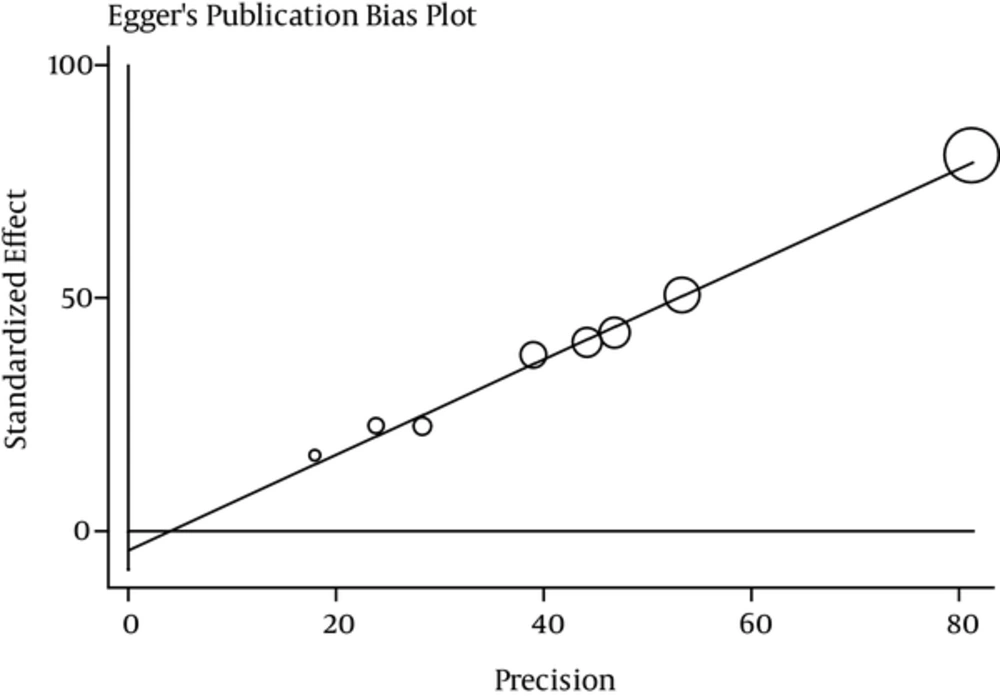
Meta-regression model was used to investigate the possible causes of heterogeneity of studies; P value was 0.964 for the year of study and 0.314 for sample size, indicating that was no statistically significant. According to Figure 5, publication bias had a significant effect on the results of these studies by showing a symmetrical funnel plot. The size of circles indicates the weight of the study; larger circles indicates larger sample sizes.
6. Discussion
The present study is the first systematic review and meta-analysis conducted to evaluate the efficacy of HBV vaccine during 1 - 6 months after the injection of the last dose of HBV vaccine among high-risk group including medical students and medical staff. The efficacy of HBV vaccine was sub-grouped by geographical region, gender, year of the study, and sample size. Iran initiated the vaccination program in 1993, and according to the studies, this program covers more than 70% of health care system personnel two decades after the implementation (8-10).
The exclusion criteria were smokers, taking immunosuppressed drugs, positive HBC antibody, positive HBs-Ag, booster dose injection, lack of full injection at 0, 1, and 6-month intervals, and non-Iranian samples. After examining 1726 HCWs in 12 studies, the efficacy of HBV vaccine, 1 - 6 months after the last dose administration was estimated as 93.1%; reports from other countries in this regard are shown in Table 5 (41-46).
| Author(s) | Country | Sample Size | Group | Age, y | Type of Vaccine | Time Elapsed Since the Last Doses | Vaccine Efficacy, % |
|---|---|---|---|---|---|---|---|
| Thakur et al. (41) | Northern India | 381 | HCW | 18 - 45 | Recombinant hepatitis B | 1 mo | 96.4 |
| Beran et al. (42) | Czech Republic | 209 | General Population | 26 - 30 | Twinrix™, GSK Vaccines, Belgium | 15 y | 81.8 |
| Thomas et al. (43) | India | 454 | HCW | 16 - 50 | Recombinant hepatitis B | 1 y | 98.1 |
| Chathuranga et al. (44) | Sri Lanka | 99 | HCW | - | - | 1 - 7 y | 86.6 |
| Yen et al. (45) | Taiwan | 250 | HCW | 25 - 70 | Recombinant hepatitis B | 8 mo | 86.4 |
| Wang et al. (46) | China | 348 | Healthy young adults | 18 - 25 | Recombinant hepatitis B | 1 mo | 97.7 |
Abbreviation: HCW, health care workers.
The type of vaccine used in the present study was recombinant which, compared to plasma-derived vaccine, had higher efficacy in the same period, although the difference between two vaccines was not significant. According to the study of Chen et al. (47), the efficacy of both recombinants and plasma-derived vaccines was similar in creating anti-HBS Ab. HBV vaccine injection mode in studies met to this meta-analysis, was intramuscular. In Chen’s systematic study, intramuscular injection at a dose of 20 mg was significantly more effective than intradermal injection at a dose of 2 mg (47).
To indicate the effect of age on HBV vaccine efficacy, sub-group analysis was conducted based on two risk groups of clinical students (aged 20 - 26 years) and medical staff (mean age of 31.98 years). The results showed that the efficacy of the vaccine was higher in clinical students and, since their confidence intervals did not intersect, this between-group difference was statistically significant. Study of Rezaee et al., conducted on Iranian children, revealed that there is no significant relationship between age and immune response (48). Other studies, such as those carried out by Chathuranga et al. in Sri Lanka (44) and Yen et al. in China (45), did not find any significant relationship between age and immune response, too. According to the present study, the efficacy of HBV vaccine was higher in male than female HCW, this difference was not statistically significant. Other studies have presented contradictory reports on the relationship between gender and HBV vaccine efficacy. For example, Thakur in India and Chathuranga et al. in Sri Lanka stated that HBV vaccine immune response was much higher in women than men (42-44). Thomas’s study did not recognize the gender of participants as an effective factor in immune response; a finding that is consistent with that of Holenger’s research indicating the weak role of gender in HBV vaccine immunogenicity (49).
In the current meta-analysis, publication bias had a significant effect on the results of the studies. Since papers providing positive results have higher chances of publication, studies that focus on relationship assessments are usually influenced by bias. However, the present study which examined the efficacy of HBV vaccine is free of such bias.
6.1. Week and Strong Points of the Study
Disregarding efficaccy of HBV vaccine based on smoking, booster dose administration, and irregular vaccination is the most important weak point of the present study; therefore, it is recommended that further meta-analysis studies investigate the effect of such factors on vaccine immune response.
On the other hand, the strong points of the study included: 1) precise estimation of HBV vaccine efficacy among originally Iranian health care staff and medical students through applying criteria which can affect immunogenicity; 2) evaluating HBV vaccine efficacy sub-groped by medical students and medical staff to show the relationship between age and HBV vaccine efficacy.
6.2. Limitations of the Study
1) There were not sufficient internal sources for searching keywords.
2) The time passed since the administration of the last dose was not mentioned in some studies, while some others did not present an accurate description of the mentioned time.
3) Some studies had investigated the effect of vaccine on immune system among all vaccinated subjects (both incomplete and complete courses of vaccination) and this caused the removal of such studies from the meta-analysis.
6.3. Conclusions
Efficacy of HBV vaccine was 90 to 97% in HCWs. Hence, the complete course of vaccination is sufficient for HBV and there is no need for booster dose or dose re-administration.