1. Introduction
Primary sclerosing cholangitis (PSC) is an inflammatory disease of bile duct epithelium. It is one of the major risk factors of cholangiocarcinoma (CCA) with reported prevalence rate of 3.5% to 36.5%. (1, 2). CCA is a cancer with an extremely poor prognosis that has a 5-year survival of less than 20% (3). However, early diagnosis of CCA in PSC patients can have a significantly positive effect on the survival rate. Recent studies have shown the spectrum of bile duct epithelial cell changes in PSC patients, indicating that different types of metaplasia and dysplasia occur with passing time that seems eventually can lead to the development of CCA (4). The above-mentioned epithelial changes in biliary epithelium are called biliary intraepithelial neoplasia (BilIN) and consist of atypical lesions classified based on nuclear atypia and loss of nuclear polarity (BiLIN-1, BilIN-2 and BiLIN-3). Diagnosis of these lesions, especially flat types, in PSC livers can be difficult (5).
In this study, we evaluated 80 formalin-fixed PSC explanted livers at our center by thorough sampling (at least 14) of large and small bile ducts in addition to routine samples. Different histologic findings in the bile duct epithelial cells including normal/reactive, metaplastic, or BiLIN-1, 2 or 3 were recorded.
Patients’ data including age, sex, MELD score and history of inflammatory bowel disease (IBD) were collected from their clinical chart. Some laboratory results of patients were also evaluated including alkaline phosphates (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (bili). Four tumor markers, i.e. carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigen-125 (CA125), and carbohydrate antigen 19-9 (CA19-9), were also recorded.
Prevalence of pathological lesions, correlation of microscopic changes (presence of bile duct epithelial changes) with gross examination of the specimens, and their correlation with laboratory findings and tumor markers were also investigated.
2. Methods
This cross-sectional study was performed in the pathology lab of Namazi hospital, affiliated to Shiraz University of Medical Sciences, on 80 explanted livers with confirmed diagnosis of PSC over a period of 2 years (2014 - 2015). The diagnosis of PSC in the explanted livers was based on clinical history, physical examination, and laboratory and radiological findings and was ultimately confirmed by pathologists. Any case with a questionable diagnosis of PSC was excluded.
Explanted livers were first evaluated grossly by careful inspection for any discoloration and presence of firm, whitish area in the background of green cholestatic liver, especially around the large bile ducts. Then, sectioning at 0.5 cm intervals was performed and each slice was inspected precisely for any mass, biliary wall thickness, firmness, and discolored nodules. A photograph was taken to compare the gross findings with microscopic characteristics. For each liver, 3 routine sections (1 right lobe and 1 left lobe and hilum) and at least 14 additional sections from central and peripheral areas were taken. Five to six additional sections were taken from the livers with any grossly visible focal lesion such as mass, discoloration, and firm area.
All of the sections were stained with Hematoxylin and Eosin, and reviewed by two pathologists. Different histopathological changes were evaluated as follows:
- Degree of acute and chronic inflammation around the bile ducts (mostly large ones)
- Bile duct epithelial ulceration
- Periductal fibrosis
- Bile sludge
- Bile duct epithelial metaplastic changes
- Bile duct epithelial dysplasia
According to the most recent classification of bile duct epithelial abnormalities, they were divided into hyperplastic or regenerative, BilIN-1(biliary intraepithelial neoplasia, low grade), BilIN-2 (biliary intraepithelial neoplasia, high grade), and BilIN-3 (carcinoma in situ) (5).
In addition to pathological findings, clinical characteristics such as age and sex, MELD score, history of inflammatory bowel disease (IBD), and duration of disease were also recorded.
Laboratory data such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin and tumor markers such as carcinoembryonic antigen (CEA) , alpha-fetoprotein (AFP) carbohydrate antigen-125 (CA125), and carbohydrate antigen 19-9 (CA19-9) were also recorded from the patients’ clinical profiles.
The statistical analysis was performed using SPSS Program version 13. The Chi-square test was applied to assess the significance of the association between the PSC histologic findings and its precursors (as variables) (1).
3. Results
During the study period, we examined 80 explanted livers from the patients with confirmed diagnosis of PSC. Demographic information is summarized in Table 1. As can be seen in the males males were affected more than females. The age range was 6 to 69 years (mean of 35.9 ± 11.8 years).
| PSC (n = 80) | PSC with BilIN (n = 9, 10.8%) | P Value | |
|---|---|---|---|
| Age, y | 35.9 (6 - 69) | 32.7 (21 - 46) | NS |
| Gender (M/F) | 35/45 | 5/4 | NS |
| Duration of PSC, mo, mean | 46.38 | 58.2 | NS |
| Positive history of IBD | 35 (56.6) | 5 (60) | NS |
| ALT, IU/L | 122 | 149 | NS |
| AST, IU/L | 130 | 121 | NS |
| Bilirubin, mg/100 | 8.8 | 4.7 | 0.01b |
| CA-125, IU/mL | 48.47 | 46.9 | NS |
| CA-19-9, IU/mL | 84.89 | 134.4 | 0.02 |
| CEA, IU/mL | 7.5 | 8.2 | NS |
| AFP, IU/mL | 2.9 | 2.6 | NS |
| MELD Score | 19.3 ± 6.5 (8 - 39) | 19.2 ± 6.6 (8 - 39) | NS |
Characteristics of PSC patients and Those with BilINa
Mean duration of disease was 46.38 months (± 54 months). Among them, 56.6% of patients had inflammatory bowel disease (IBD) that had been confirmed by colonoscopy and colon biopsy. MELD score was 19.3 ± 6.5 (in range of 8 - 39).
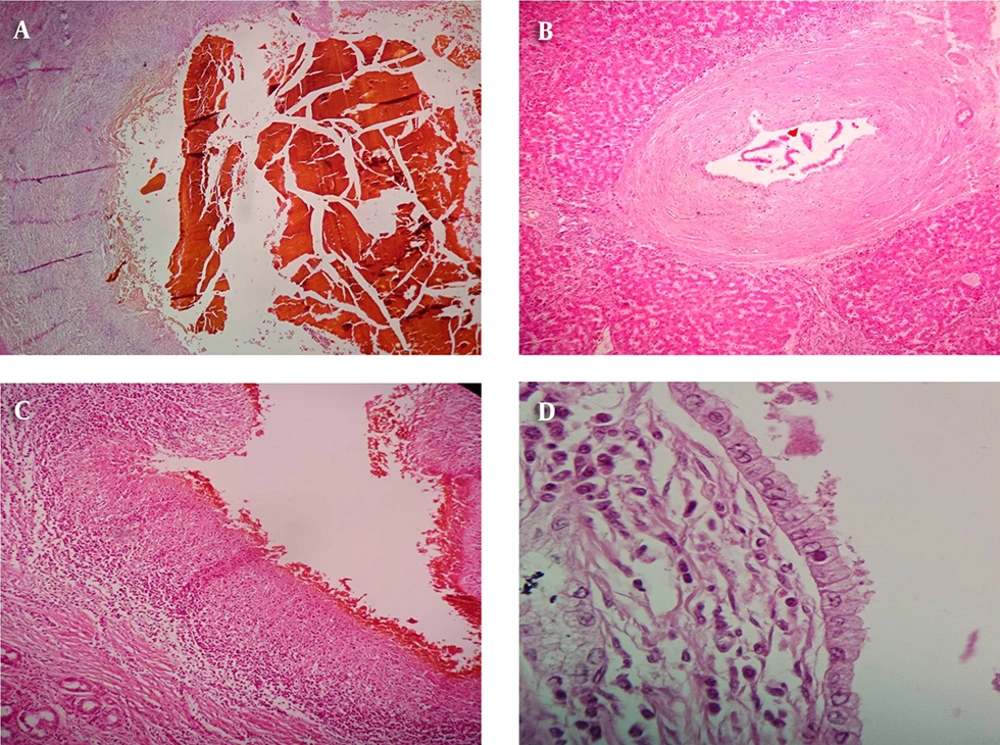
In gross examinations, spectrum of changes was observed and it was revealed that most of the cases showed green color with large amount of bile sludge in dilated bile ducts while some cases showed creamy-green color with firmness around narrowed bile ducts and a small amount of bile sludge (Figure 1).
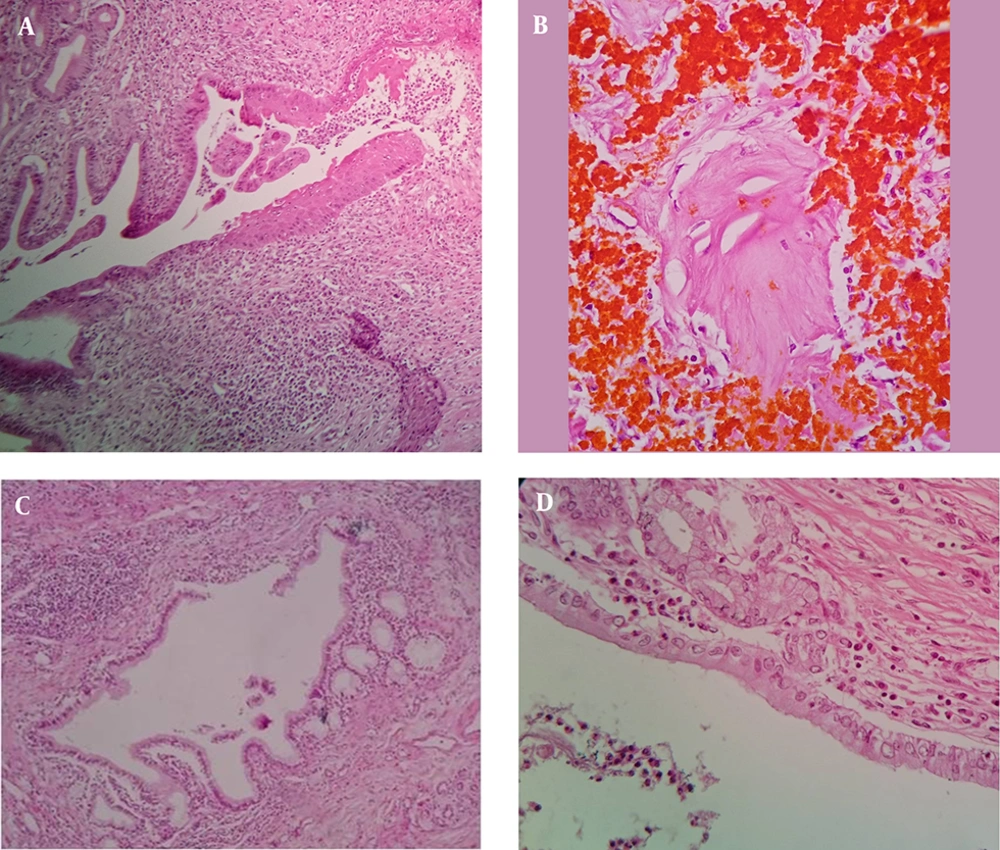
Study of H & E slides showed (Figures 2 and 3):
- Pyloric, mucinous (BilIN-1), intestinal, osteoid, and squamous metaplastic changes were identified in order of frequency (Table 2).
- BilIN-1 (mucinous metaplasia) was found in 9 cases of PSC (10.8%).
- Two of the above-mentioned patients with BilIN-1 had also intestinal metaplasia.
- None of the studied cases had criteria for BilIN-2 and 3.
A, bile sludge which means concentrated inspissated bile within a dilated bile duct with denuded and sloughed epithelium; B, periductal onion skin like fibrosis which is the characteristic histologic finding in PSC; C, bile duct epithelial ulceration; D, mitotic figures in the bile duct epithelium.
4. Discussion
PSC as a chronic cholestatic liver disease is characterized by long-standing and progressive inflammation and fibrosis around the bile ducts. This disease can lead to liver cirrhosis and may be superimposed by bile duct epithelial changes (6).
There are controversial reports about the multistep carcinogenesis in PSC, i.e. the sequential steps of inflammation/hyperplasia/metaplasia/dysplasia in the course of development of cholangiocarcinoma in the patients with PSC (4, 7-10).
Diagnosis of these lesions as precursors of malignancy in PSC livers can be difficult. Imaging techniques, although have essential role in diagnosis of CCA, are not helpful to detect flat precancerous epithelial lesions. Thus, diagnosis should be based on pathological and laboratory information (10).
Very few studies have been performed to find the incidence of biliary intraepithelial neoplasia (BilIN) as precursor lesions of invasive adenocarcinoma in the biliary tracts (cholangiocarcinoma); most of the previous studies on PSC-associated bile duct epithelial dysplasia have been in the form of case reports and there are only a few case series (7-16).
In this study conducted on 80 PSC formalin-fixed explanted livers during 2 years (2014 - 2015), there were 43(53.75%) PSC cases with different types of bile duct epithelial metaplasia. Pyloric type metaplasia was the most frequent type of metaplasia, which has been found in 29 cases (35%) of PSC, followed by mucinous metaplasia (which is classified as BilIN-1) with 10.8% prevalence. This finding is compatible with that reported by Abraham et al. in 2010. They studied 30 explanted livers with PSC and found mucinous metaplasia and pyloric metaplasia as the two most frequent epithelial changes in 77% and 73% of livers, respectively (4). In 2 out of 9 patients (22%) with BilIN-1, we also observed intestinal metaplasia.
In our study, there were 2 cases with unusual metaplastic changes such as squamous (in one case with 24 months duration of disease) and osteoid (in one case with 48 months duration). Abraham et al. noticed squamous metaplasia in 3% of their cases; however, osteoid metaplasia was not reported in the previous studies. On the other hand, we did not have any case with pancreatic acinar metaplasia, which has been reported in 10% of the previously examined cases (4).
Our data showed frequent metaplastic changes in the bile duct epithelium of PSC livers but dysplastic changes (low and high) were not found.
In PSC group, Bergquist et al. found 2 cases out of 16 (13%) (8), Ludwig et al. found 1 case of papillary hyperplasia among 60 (3%) (17), Fleming et al. found no case (10) and Katabi and Albores-Saavedra found only 2 (4%) cases with dysplastic changes (18). However, there were also studies with higher frequencies of bile duct epithelial changes (4). Abraham et al. reported 36% dysplasia in their study population. It should be noted that Abraham et al. worked on explanted livers and took more sections especially from large bile ducts (4); but other studies used needle biopsy or if they worked on whole livers, they had limited number of sections (8, 17, 18). In this study, we worked on whole explanted liver with thorough sampling (at least 14 from peripheral and central bile ducts); however, no dysplasia was found. (Table 3 shows the frequencies of bile duct epithelial changes in different studies). According to the previous studies conducted at our center, compared to other centers in the Western parts of the world, the incidence of CCA in our PSC cases is low to intermediate. This means that the incidence of CCA has been reported in up to 36.5% of PSC patients, while it has been 8.8% at our center (6). The absence of any bile duct epithelial dysplasia in 80 PSC livers with mean disease duration of around 4 years is compatible with low incidence of CCA in our PSC cases.
| Author | Year | Country | Specimen | No. of cases | Disease | Metaplasia, % | Dysplasia, % | ||
|---|---|---|---|---|---|---|---|---|---|
| BilIN 1 | BilIN 2 | BilIN 3 | |||||||
| Lewis et al. (4) | 2010 | USA | Explanted liver | 30 | PSC | 83 | |||
| Mucinous, 4 | |||||||||
| Pyloric, 73 | |||||||||
| Intestinal, 26 | |||||||||
| Pancreatic, 10 | |||||||||
| Wu et al. (19) | 2009 | USA | Explanted liver | 244 | NR | ||||
| Noncirrhotic | 40 | 16 | 0 | ||||||
| EtOH | 35 | 57 | 4 | ||||||
| EtOH + HCV | 38 | 54 | 4 | ||||||
| HCV | 55 | 20 | 7 | ||||||
| Sato et al. (20) | 2013 | Japan | Resected liver | 64 | PSC | NR | 30 | 7 | 0 |
| Fleming et al. (10) | 2001 | UK | Liver Bx | 60 | PSC | NR | 0 | ||
| Bergquist et al. (7) | 1998 | Sweden | Explanted liver | 20 | CCA | NR | 60 | ||
| Ludwig et al. (17) | 1992 | USA | Explanted liver | 60 | PSC | NR | 1.25 | ||
| Current Study | 2015 | Iran | Explanted liver | 80 | PSC | 0 | |||
| Pyloric 35 | |||||||||
| Mucinous 10.8 | |||||||||
| Intestinal 3.6 | |||||||||
| Osteoid 1.2 | |||||||||
| Squamous 1.2 | |||||||||
Comparison of the Frequencies of Different Epithelial Changes in Bile Ducts of the Patients with PSC in Different Studies
Patients with PSC usually are followed up by imaging techniques and regular monitoring of tumor markers in addition to other laboratory tests to diagnose the development of CCA as soon as possible. However, many cases with CCA are detected late in their course with poor prognosis. In early stages, cytology/biopsy and imaging studies are usually negative (21).
Diagnosis of PSC with bile duct epithelial metaplasia and dysplasia as early precursors of malignancy can be important to increase patients’ survival. In our study population, there were no significant demographic differences between PSC cases and those PSC cases with BilIN; however, bilirubin was significantly higher in the former group. In the study performed by Haseeb et al. the level of bilirubin was introduced as a predictor of CCA; however, there is no report about the level of bilirubin and development of bile duct epithelial damage in PSC patients (22).
In our study group consisted of 80 patients with PSC, most of the tumor markers were the same in the livers with and without BilIN, however, CA19-9 was significantly higher in the former group. Although this relationship has not been investigated before, the level of CA19-9 has been recognized as a good predictor of CCA in the patients with PSC (21).
4.1. Conclusion
The frequency of bile duct intraepithelial neoplasia (dysplasia) in Iranian patients with PSC is low.
Bilirubin and CA19-9 can be considered as possible predictors of BilIN in patients with PSC to detect early development of CCA.