1. Background
The hepatitis B virus (HBV) represents a worldwide public health problem. According to the world health organization (WHO), two billion people worldwide are positive for serological markers of HBV, of whom more than 240 million are chronically infected (1). The high rate of chronic HBV infection in China is mainly caused by intrauterine infection or early childhood transmission. Hepatitis B virus vaccines have been applied for universal neonate and early childhood vaccination worldwide and have led to a 70 - 90% decrease in chronic HBV carrier rates (2). In China, administration of universal HBV vaccination in infants has led to a dramatic decline in HBV positive rates, with hepatitis B surface antigen (HBsAg) prevalence decreasing from 9.75% in 1992 to 7.18% in 2006 (3). Moreover, data have shown that the prevalence of HBV has consistently decreased in the recent years (4). However, the HBV vaccine cannot eliminate emerging HBV infection. Approximately 10 - 30% of newborns from HBsAg/hepatitis B envelope antigen (HBeAg)-positive mothers cannot be protected by passive/active vaccination alone and become chronic HBV carriers themselves. Asymptomatic Occult HBV infections (OBIs) are frequent, even in those who have protective levels of hepatitis B surface antibody (anti-HBs) (2). In China, 5 - 10% of the population shows little or more response after HBV vaccination. Even after newborn immunoprophylaxis, vertical transmission still accounts for 5% of cases of HBV infection in China (5). Additionally, immunosuppressive patients and patients infected with HIV or other viruses are considered susceptible to HBV. Thus, HBV infection is still a serious problem. Currently, about 0.40% of donated blood from apparently healthy donors in Jiangsu province blood center in east China is HBsAg positive and is hence discarded. However, this rate may be higher due to OBI, as shown by nucleic acid testing (NAT), implemented at our center in the beginning of 2010.
Occult HBV infections was defined at an international workshop in Italy in 2008, as the presence of circulating HBV DNA detected by HBV NAT, with or without detectable antibody against the core antigen (6). This became evident when patients, who received blood transfusions from HBsAg-negative donors, went on to develop overt infection. In 2008, when NAT was recommended for blood screening, more cases of OBI were found worldwide (7). In 2010, NAT was piloted at 10 blood centers in China, including Jiangsu province blood center. Epidemiological data regarding the global prevalence of OBI vary because the detection methods used have different sensitivities and specificities in different regions.
2. Objectives
In this study, we evaluated the prevalence and characteristics of OBI from samples collected at our blood center in east China during years 2013 and 2014.
3. Methods
3.1. Sample Collection
All blood donors were determined to be eligible through a health history questionnaire, a brief physical examination, and predonation rapid testing for HBsAg, syphilis, ABO blood type, and hemoglobin. Donors, who initially tested negative for HBsAg and syphilis, were eligible to donate. This study was carried out on predonation-eligible samples at our blood bank from January 2012 to December 2013. After routine screening by two different enzyme-linked immunosorbent assay (ELISA) kits (dual-ELISA) for anti-human immunodeficiency virus (HIV)-1, anti-HIV-2, anti-hepatitis C Virus (HCV), Treponema pallidum (TP), and HBsAg, samples with reactive results in any one ELISA were considered to be reactive. All ELISA reactive samples were discarded and the donors were deferred permanently. Total HBsAg nonreactive, alanine aminotransferase (ALT)-elevated, and HBsAg single-reaction samples were collected for further NAT. All donors provided written informed consent for the use of their samples for research purposes before donation. This study was approved by the ethics committee of Jiangsu province blood center (approval number 2-2012/3/6) and all study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
3.2. Nucleic Acid Testing
All negative samples were screened by the Roche Cobas TaqScreen MPX test on the Cobas S 201 system (Roche molecular systems, Branchburg, NJ, USA; MPX test); the MPX test is a multiplex NAT test for the simultaneous detection of HBV DNA, HCV RNA, and HIV RNA in human plasma. We used Roche Ampliscreen polymerase chain reaction (PCR) assays (MP-NAT; pool size of 6 × 100 μL; Roche molecular diagnostics, Raritan, USA). The MP6-pool NAT yielded samples were resolved and tested by individual NATs (ID-NATs) to determine which donors were positive in MP6-NATs.
3.3. Discriminatory Tests for ID-Nucleic Acid Testing Yield Samples
The ID-NAT yield donations were tested further with the discriminatory COBAS AmpliPrep/COBAS TaqMan HBV test, COBAS AmpliPrep/COBAS TaqMan HCV test, and COBAS AmpliPrep/COBAS TaqMan HIV test (Roche Molecular Systems) on a Cobas TaqMan analyzer (Roche Diagnostics Company, Shanghai, China). The manufacturer states that the lower limit of detection for the HBV DNA assay is 20 IU/mL. Hepatitis B virus DNA of follow-up samples was tested for viral load by the same method.
3.4. Supplemental Test for Seromarkers of Hepatitis B Virus
Supplemental testing of HBV seromarkers (HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc) was performed using Electrochemiluminescence Immunoassays (ECLIAs) with a Cobas e601 analyzer (Roche diagnostics company). Follow-up samples were taken from donors with an HBV DNA NAT reactive result and tested for all HBV serologic markers with AmpliPrep/TaqMan HBV v 2.0 tests.
3.5. Molecular and Phylogenetic Analyses
HBsAg-/DNA+ samples were collected and stored at -80°C. The donors were followed up and blood samples were recollected. DNA was extracted from 200 μL of serum, using a Qiagen DNA blood mini kit (Qiagen, Venlo, the Netherlands), according to the manufacturer’s instructions. The S gene from the HBV genome was amplified by nested PCR. Viral DNA was extracted from 200 μL of plasma using a QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). The first round of PCR was performed using an outer primer set (5’-ACTGTCTCTGCCATATCGTCA-3’, 5’-CCAACACCCAATTACATATC-3’) for 38 cycles (94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 40 seconds). The second round was performed using an inner primer set (5’-ATGGAGAACATCGCATCAGG-3’, 5’-TTAAATGTATACCCAAAGAC-3’) for 38 cycles (94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds). Polymerase Chain reaction products were sequenced directly. Phylogenetic analyses were performed using MEGA v.5 (Tempe, AZ, USA) and the neighbor-joining method of the Kimura 2-parameter model with 1000 bootstrap replicates.
3.6. Statistical Analyses
A computerized data sheet was used for record keeping; all data were evaluated with SPSS 17.0 for Windows Statistical Software Package (SPSS Inc., Chicago, IL, USA). Results with P-values (two-tailed) of less than 0.05 were considered statistically significant.
4. Results
4.1. Routine Screening Results of Blood Samples
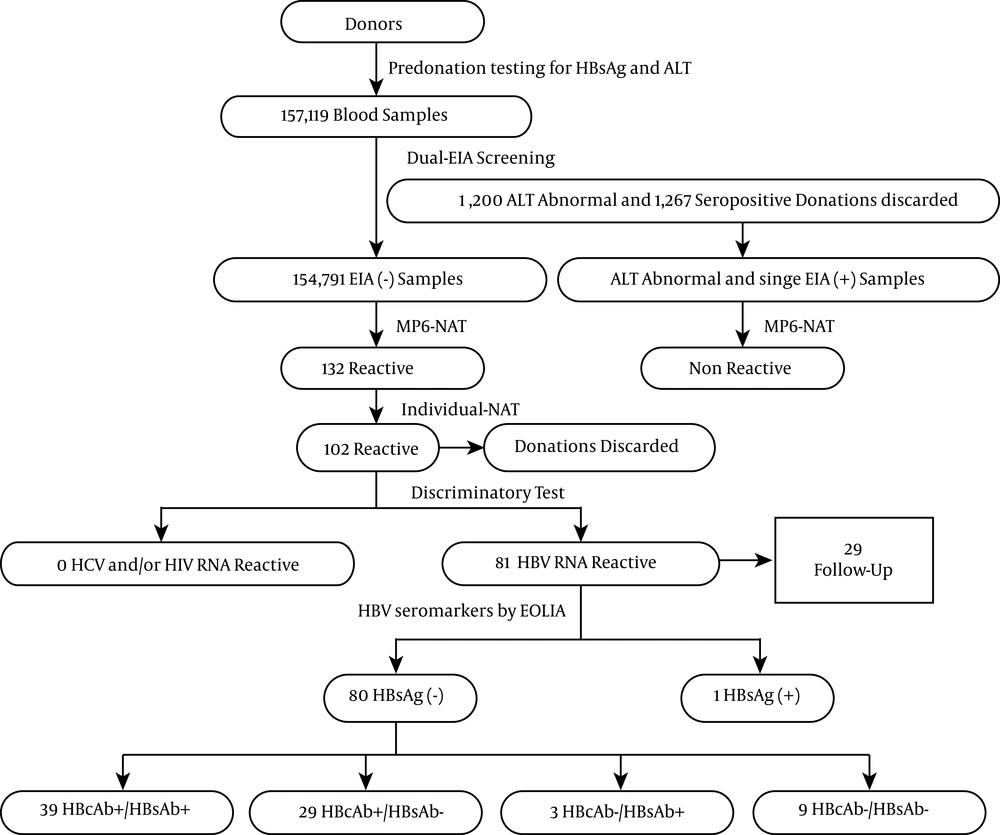
A total of 157119 blood samples were collected from January 2013 to December 2014. After dual-ELISA tests for anti-HIV, anti-HCV, HBsAg, and anti-TP and biochemistry assays for ALT, 1200 samples that had elevated ALT (> 40 IU/mL), 498 samples that were HBsAg reactive (433 dual-reactive and 85 single-reactive), 347 samples that were anti-HCV reactive, 283 samples that were anti-TP reactive, and 139 samples that were anti-HIV reactive were discarded. In our district, the prevalence of HBsAg was 0.32% in blood donors.
Next, 154791 HBsAg, anti-HIV and anti-HCV nonreactive samples with normal ALT, including 55829 samples (36.1%) from repeat donors and 98823 (63.9%) from first-time donors, were screened for HBV DNA, HIV-1, and HCV RNA by 6-pool-NAT; 132 pools were reactive (NAT yield). Eighty-one samples were reactive after analysis by ID-NAT; these samples were all HBV DNA-positive, with no HIV or HCV RNA-positive samples. In order to verify whether elevated ALT and single-ELISA reactive samples were truly infected, samples were screened by NAT together with nonreactive samples. For these samples, no NAT-reactive samples were detected among the elevated ALT and single-ELISA reactive samples. The initial yield of 81 HBV DNA+/HBsAg- samples was confirmed by ECLIA. Only one sample was positive. The total HBV NAT yield rate was 1 in 1935 (80/154791) among HBsAg nonreactive blood donors.
4.2. Serological Patterns in Hepatitis B Virus DNA+/HBsAg- Donors
Next, we detected other HBV seromarkers (Table 1). The mean age of 80 HBsAg-/DNA+ donors was 36.9 ± 10.5 years (21 to 60 years), and there were 62 males and 18 females (ratio = 3.4:1). There were no significant differences between first-time and repeat donors in either OBI frequency or gender. A significant difference in age distribution was observed among the four serologic groups, with anti-HBc-positive individuals being significantly older (P < 0.05). Only one individual had elevated ALT (48.4 IU/L); all others had normal ALT values (range: 4.2 - 37.2 IU/L). Of the blood donors, 85.0% (68/80) were anti-HBc positive, of which 39 (48.8%) were anti-HBs positive and 29 (35.4%) were anti-HBs negative. Twelve blood donors were anti-HBc negative, whereas three and nine were anti-HBs positive and anti-HBs negative, respectively. Of the nine anti-HBc-negative/anti-HBs-negative donors, three were anti-HBe positive. Of the 32 anti-HBs-positive donors, only two (6.3%) had anti-HBs levels of less than 10 IU/L at their index donation, and six (18.7%) had anti-HBs levels of more than 100 IU/L; the remaining 24 (75%) had levels between 10 and 100 IU/L. No significant differences were observed between HBV DNA positivity and anti-HBs levels.
| Markers | Anti-HBc(+), Anti-HBs(-) | Anti-HBc(+), Anti-HBs(+) | Anti-HBc(-), Anti-HBs(+) | Anti-HBc(-), Anti-HBs(-) |
|---|---|---|---|---|
| No. (%) | 39 (48.8) | 29 (36.2) | 3 (3.8) | 9 (11.2) |
| First/repeat (47/33) | 27/12 | 14/15 | 0/3 | 6/3 |
| Gender, F/M, 18/62 | 6/33 | 6/23 | 1/2 | 5/4 |
| Age, Range (median), y | 21 - 58 (40.7) | 28 - 60 (42) | 28 - 44 (36) | 21 - 45 (28.6) |
| ALT, Range (median), IU/mL | 4.2 - 35.5 (18) | 6.3 - 48.4 (22) | 7.7 - 21.4 (12.6) | 7.2 - 37.2 (20.2) |
4.3. Follow-up Donors
Twenty-nine DNA+ donors were followed up successfully, all of whom were anti-HBc positive, but not HBsAg positive (Table 2). Donor 037 was negative by HBsAg EIA and positive by ECIA; her viral load was 2500 IU/mL, and other seromarkers were all negative. A month later, her tests showed DNA negative, HBsAg negative, and anti-HBs/HBc positive results, suggesting that her infection was acute. Six donors, i.e. 027, 058, 061, 063, 071 and 076, had high index viral loads (> 1500 IU/mL), but all seromarkers were negative. With the exception of 061 and 071, these donors were followed up for different time intervals. These individuals were anti-HBs/HBc positive, DNA negative, and HBsAg negative. Donors 041 and 056 were repeat donors who had the same serum pattern (anti-HBs/HBc positive) and were DNA positive from index to follow up, and their anti-HBs did not protect them from HBV infection. Donor 065 was anti-HBs/HBc positive; however, after 164 days, the donor was tested as anti-HBs negative and DNA negative. The seromarkers of other donors were not changed although DNA was negative for most donors at follow up. Overall, from the 80 samples, six may have been collected during the HBV infection window, and the others had OBIs. Thus, the prevalence of OBI was 0.047% (74/157,119; 1:2123).
| No. | Age | Gender | Initial Screening | Follow-Up Screening | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT | HBsAg S/CO | 6-Pool Ct | ID Ct | HBsAg | Anti-HBs | HBeAg | Anti-HBe | Anti-HBc | Days | ALT | HBV VL, IU/mL | HBsAg | Anti-HBs | HBeAg | Anti-HBe | Anti-HBc | |||
| 005 | 27 | M | 13.6/13.3 | 0.020/0.019 | 46.2 | 43.3 | — | — | — | + | + | 80 | 23.8 | — | — | — | — | + | + |
| 008 | 45 | M | 18.5/19 | 0.038/0.057 | 24.4 | 44.4 | - | - | - | + | + | 100 | 46.5 | < 20 | - | - | — | + | + |
| 009 | 31 | M | 16.3/16.8 | 0.077/0.029 | 52.1 | 37.8 | - | - | - | + | + | 107 | 14.1 | - | - | + | - | + | + |
| 010 | 44 | M | 32.8/35.5 | 0.010/0.048 | 39.6 | 36.6 | - | - | - | + | + | 95 | 28.4 | < 20 | - | - | - | + | + |
| 013 | 40 | M | 23.6/22.2 | 0.029/0.067 | 38.4 | 34.9 | — | - | - | + | + | 77 | 22.9 | < 20 | - | + | - | + | + |
| 016 | 46 | M | 5.1/4.2 | 0.029/0.067 | 38.0 | 40.9 | - | - | - | + | + | 75 | 30.1 | 66.6 | - | + | - | - | + |
| 022 | 44 | M | 17.6/17.3 | 0.083/0.150 | 34.5 | 32.2 | - | - | - | + | + | 75 | 13.7 | 27.2 | - | + | - | - | + |
| 023 | 27 | M | 3.7/5.7 | 0.185/0.029 | 40.6 | 35.1 | - | - | - | + | + | 65 | ND | — | - | - | - | + | + |
| 027 | 27 | F | 31.1/37.2 | 0.049/0.181 | 29.2 | 27.1 | - | - | - | - | - | 75 | 20.3 | — | - | + | - | - | + |
| 029 | 55 | M | 10.1/9.7 | 0.040/0.038 | 43.2 | 39.4 | - | + | - | - | + | 60 | 124 | -— | — | + | — | — | + |
| 033 | 51 | M | 11.1/10.8 | 0.139/0.038 | 39.5 | 53.1 | - | - | - | - | + | 81 | 32.7 | — | - | + | - | + | + |
| 037 | 31 | F | 23.2/26.2 | 0.057/0.295 | 29.7 | 26.9 | + | — | — | — | — | 30 | ND | — | — | + | — | — | + |
| 041 | 28 | M | 22.4/23.7 | 0.059/0.219 | 28.0 | 25.4 | - | + | - | + | + | 30 | 74.3 | 538 | - | + | - | + | + |
| 044 | 39 | M | 25.1/26.3 | 0.029/0.029 | 33.7 | 30.9 | - | + | - | + | + | 35 | 3 | 2321 | - | + | - | + | + |
| 047 | 44 | M | 25.1/26.3 | 0.029/0.029 | 33.1 | 30.0 | - | + | - | - | - | 80 | ND | — | - | + | - | + | + |
| 048 | 42 | M | 24.4/26.6 | 0.010/0.038 | 33.7 | 42.7 | - | + | - | - | + | 65 | ND | — | - | + | - | - | + |
| 049 | 44 | M | 22.8/24 | 0.126/0.276 | 36.9 | 35.5 | - | + | - | + | + | 43 | ND | — | — | + | — | + | + |
| 052 | 39 | F | 14.2/13.9 | 0.070/0.057 | 31.6 | 52.8 | — | + | — | — | + | 55 | 10.5 | — | — | + | — | — | + |
| 055 | 27 | M | 13.6/13.3 | 0.020/0.019 | 46.2 | 43.3 | - | - | - | + | + | 78 | 9.1 | < 20 | - | - | - | + | + |
| 056 | 38 | M | 19/23 | 0.070/0.057 | 33.2 | 30.9 | — | + | — | + | + | 30 | 18 | 1470 | — | + | — | + | + |
| 058 | 24 | F | 16.3/16.8 | 0.020/0.105 | 38.0 | 34.3 | — | — | — | — | — | 35 | 23.6 | — | — | + | — | - | + |
| 063 | 21 | F | 9.9/8.2 | 0.250/0.371 | 37 | 27.4 | — | — | — | — | — | 56 | 32.1 | — | — | + | — | + | + |
| 065 | 42 | M | 15.8/17.8 | 0.040/0.067 | 36.8 | 39.4 | — | + | — | — | + | 164 | 28.1 | — | — | — | — | — | + |
| 066 | 57 | M | 15.8/17.8 | 0.110/0.038 | 35.3 | 31.3 | — | + | — | — | + | 95 | 30.5 | — | — | + | — | — | + |
| 074 | 48 | M | 19.2/22 | 0.020/0.124 | 36.7 | 35.2 | - | - | - | + | + | 14 | 16,7 | 121 | - | - | - | + | + |
| 076 | 21 | F | 8.9/9.8 | 0.095/0.162 | 37 | 27.4 | — | — | — | — | — | 107 | ND | — | — | + | — | + | + |
| 077 | 48 | M | 14.5/16.3 | 0.076/0.042 | 40.1 | 35.8 | - | - | - | + | + | 104 | 23 | 84.5 | - | - | - | + | + |
| 078 | 49 | M | 7/6.3 | 0.120/0.029 | 54.4 | 35.5 | — | + | — | — | + | 7 | ND | 104 | — | + | — | — | + |
| 084 | 48 | M | 22.1/24.3 | 0.295/0.440 | 36.7 | 35.2 | — | — | — | + | + | 55 | 24.6 | 120 | — | — | — | + | + |
4.4. Molecular Characterization of the S Gene Among Hepatitis B Virus DNA+/HBsAg- Samples
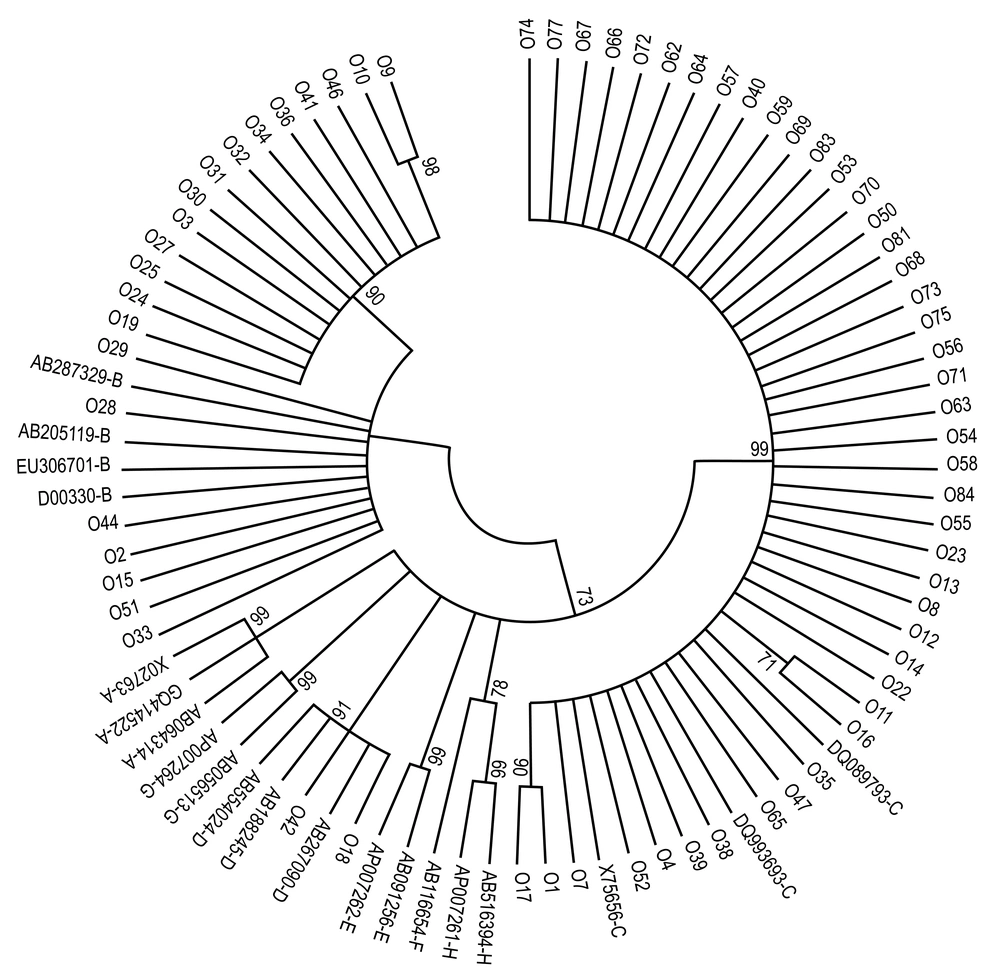
Hepatitis B Virus DNA from 81 HBsAg-/DNA+ was amplified in the HBV S region. Seventy-one samples were sequenced successfully and were subsequently analyzed using Mega 6.0 software with HBV reference sequences. Three genotypes, including 29.6% (21/71) genotype B, 62.0% (44/71) genotype C and 2.8% (2/71) genotype D, were found by phylogenic tree construction (Figure 2); the genotype could not be identified for 5.6% (4/71) of samples. Regarding the S protein, all genotype B strains were serotype adw2, all genotype C strains were adrq+, and two strains (one adw3 and one ayr) were genotype D. Thus, in our blood center, genotype C and subtype adrq+ were dominant.
Next, we constructed a phylogenetic tree of the S gene from the HBV genome. Phylogenetic analysis was inferred from distance analysis (Kimura 2 parameters model), and neighbor-joining reconstruction from the S gene of OBI sample sequences showed that HBV sequences clustered in the HBV genotype E branch. Hepatitis B Virus sequences were referred to by numbers, i.e. “016.” The HBV sequences were compared with the HBV reference sequences for the eight HBV genotypes (NCBI-GenBank accession numbers are shown in Figure 2). The numbers at the nodes indicate bootstrapping values as a percentage of 1000 replicates.
4.5. Mutations in HBsAg in the Major Hydrophilic Region (MHR) and “a” Determinant
In this study, only a few mutations were observed in the S protein when compared with consensus OBI strain sequences with other reported non-OBI strain reference sequences. The MHR (amino acids 103 - 173) and “a” determinant region (amino acids 124 - 147) in the S protein were relatively conserved, and no hot-spot mutations were found. Variants were found in 30.4% (21/69) of cases with OBI and included S114T, G119R, P120S, T125M, C139Y, T140I, C147W, T148A, A159V/G, E164D, V168A and R169C. The well-known G145A/R variant was not observed.
5. Discussion
The relatively high prevalence of OBI is relevant in areas where HBV infections are endemic worldwide, and OBI represents a major threat to blood safety. Therefore, performing HBsAg tests alone does not completely eliminate the risk of HBV transmission to blood recipients. The OBI is associated with the presence of anti-HBc or anti-HBs. Additionally, in some cases, neither anti-HBc nor anti-HBs can be detected (8). Given the above considerations, implementation of NAT worldwide, regardless of whether the prevalence is high or low, could lead to substantial improvements in safety, particularly during the window period and to prevent transfusion-related transmission of hepatitis B owing to OBI.
Nucleic acid testing has been introduced in many countries as a routine screening method and has a detection limit ranging from 1:1000 to 1:50000, depending on the epidemiology and sensitivity of the assay (9). Nucleic acid testing yields are detection of HBV DNA as a marker of HBV infection when HBsAg is absent in blood. These NAT yields for HBV can be of two types based on the presence of anti-HBc. The NAT yield without anti-HBc is thought to represent the window period phase of the infection (WP yields), whereas yields with anti-HBc are considered to represent OBI. In the current study, in 80 HBsAg-negative samples, six were Window Period (WP) infection, and the remaining 74 were OBI; thus, the incidence of OBI among Jiangsu individuals was 0.047% (1:2123). The prevalence of OBI varies to a great extent in different countries, depending on a number of factors, such as HBV endemicity, liver disease, HBV screening method, and primers employed for NAT. In China, because different screening methods are used before donation at different blood centers, and because different HBsAg assays are used, with or without confirmation of HBsAg-negative samples, the prevalence of OBI has been reported to vary dramatically. In Nanjing, the same city in which our blood center is located, the positive rate of OBI was 0.13% (5 of 2972), as determined by nested-PCR of plasma samples delivered to a hospital before implementation of NAT and without HBsAg confirmation (10). In the southeast of China, the prevalence of OBI was 0.2% by HBsAg-negative confirmation and follow-up tests (11). Additionally, the prevalence of OBI in Taiwan was 0.1% (from 10727 seronegative blood donors) (12), whereas in Hong Kong this was reported as 0.13% (4/3044) and 0.11% (11/9967) for two cohorts (13). However, in other studies, after introduction of a more sensitive transcription-mediated amplification assay, the HBV NAT-yield rates of OBI were 1:5120 and 1:2450 by ID-NAT using Ultrio and Ultrio Plus assays (Novartis Diagnostics), respectively (P < 0.0001) (14).
The results of similar studies have shown that there is great variation in anti-HBc-positive blood donors, with frequencies of 0% in Lao PR (15) and Iran (16) and 38% in Japan (17). Recently, in the HBV endemic region of Laos, a high OBI prevalence of 10.9% was reported among blood donors, who were HBsAg negative and anti-HBc and/or anti-HBs positive (15). In North Africa, a study conducted among blood samples from 1026 Egyptian donors revealed that 8% were reactive for anti-HBc and 0.5% were positive for HBV-DNA (18). In this study, we did not detect anti-HBc in all HBsAg samples; therefore, the prevalence of DNA+ in HBsAg-/anti-HBc+ samples was not determined.
In China, the genotypes of HBV in blood donors differ depending on region. For example, genotypes A and D are rare in China, whereas genotype B and C are predominant, with genotype B being more frequent in the southern part of China and genotype C being more frequent in the northern part of China (19). Yong-Lin et al. (20) investigated 39 HBsAg-positive blood donors from our city; 32 strains (82.1%, 32/39) were classified as genotype B, while seven strains (17.9%, 7/39) were classified as genotype C. No other genotypes were observed. In our OBI samples, the prevalence of genotype C was significantly higher than genotype B in donors with OBIs. This result was the same as that in donors from southeast (21) and northeast China (11). Interestingly, we observed two genotype D strains, which are usually detected in Central Asia, Russia, Inner Mongolia and Africa (22). Unfortunately, we were unable to investigate the infection factors for these two donors because they were not available for follow up.
In a previous analysis of amino acid mutations in HBsAg-positive blood donors, the mutation ratio was found to be over 50% (51.3%, 20/39) (20). In contrast, in our OBI samples, 30.4% (21/69) of samples harbored mutations, and no G145R was found. The well-known G145R mutation is the major variation in HBV isolates responsible for OBI in southeast China (21). However, in OBI samples from northeast China, no hot-spot mutations have been found, and MHR is relatively conserved. Further studies are needed to determine the effects of these variations on HBsAg and HBV.
Mu et al. (23) found that the prevalence of OBI among HBV-vaccinated children in Taiwan was 10.9%. In the healthy general population, about 5% of individuals had little or no reaction to the HBV vaccine (24). One long-term follow-up study reported that, of 2919 Chinese young adults vaccinated as infants, 2.1% exhibited chronic HBV, whereas 4.2% had OBI at age 19 to 21 years (25). In 80 initial HBsAg-/DNA+ blood donors, 40% (32/80) were anti-HBs positive and possessed detectable levels of HBV-DNA; of these individuals, eleven, who were followed-up, provided anti-HBs-positive samples, and four had a high viral load. These results were consistent with a study by Zheng et al. (26), who showed that of 14 HBV DNA+ vaccinated donors, seven had high levels of anti-HBs. These findings may be explained in part by the findings of Levicnik-Stezinar et al. (27), who concluded that low levels of anti-HBs (< 100 IU/L) have limited neutralizing capacity. This may also reflect the “natural boosts” reported in a study from Thailand (28), highlighting the need for studies of the prevalence of OBI in individuals, who were vaccinated as adults. Hepatitis B Virus-vaccinated blood donors, who were DNA-positive will be followed up in studies of the molecular characterization of HBV.Because of the cost of NAT, our blood center uses MPX tests, which allow the simultaneous detection of HBV, HCV, HIV-1 and HIV-2 with a mixture of six samples. Some studies have suggested that MPX may not identify samples with low viral load because of sample dilution. Minipool testing (minipools of 4, 8, and 16 donations) may not identify 43 - 79% of HBV-yield donations, and compared with simulated minipool-NAT, ID-NAT may be a more sensitive NAT strategy in regions of high HBV endemicity (29). In Italy, highly sensitive HBV DNA detection methods showed that 6-MP HBV DNA screening failed to identify 14/28 (50%) viremic donations, which were released for transfusion (30). However, two studies (31, 32) from the Chinese national center for clinical laboratories (NCCL) demonstrated that despite the dilution factor, the MP6-TaqScreen system detected a significantly higher proportion of qPCR-confirmed HBV NAT yields than the Ultrio ID-NAT algorithm (1/1590 versus 1/2488; P < 0.01). Notably, the Ultrio assay detected genotype B and C strains in China with reduced analytical sensitivity. Blood donation screening by NAT is now compulsory in mainland China, and more OBI data are needed to evaluate the efficacy of NAT nationwide.