1. Background
Autoimmune hepatitis (AIH) is a chronic liver disease characterized by inflammation, interface hepatitis, hypergammaglobulinemia, and production of antibodies (1). It has been recommended that decisions about treatment continuation, intensification or withdrawal of immunosuppressive therapy should be based on histological features and clinical remission (2). Liver biopsy is, however, an invasive method with possible complications (3).
The need to develop non-invasive, accurate, and complication-free methods to assess the degree of fiibrosis has led to the development of numerous non-invasive markers based on laboratory markers or technical methods such as transient elastography (TE) (4). Most of the non-invasive markers such as ratio of AST to ALT, APRI, and FIB-4 (5-9) have been established not only in large collectives with viral hepatitis, but also in collectives with NASH (10). These markers use laboratory tests that are easy and cheap to obtain and are often measured as part of the routine laboratory assessment in patients with chronic liver diseases. In a study from Abdollahi et al. (11) conducted on patients with AIH, the performance of laboratory non-invasive markers (ratio of AST to ALT, APRI, and FIB-4) was evaluated in comparison with liver biopsy. All calculated non-invasive markers performed unsatisfyingly in patients with AIH. Other smaller studies have indicated, however, that predicting degree of fiibrosis in patients with AIH using laboratory parameters or transient elastography is feasible, and platelet count and AST/ALT-ratio can be used to predict the presence of advanced fiibrosis (12-15). Furthermore, in a Chinese study, comparing diagnostic performance of TE and liver biopsy in 30 patients with AIH, liver stiffness measured by TE correlated significantly with the stage of liver fiibrosis, and in another study TE performed better than non-invasive markers (16, 17). It has been demonstrated that TE can accurately predict fiibrosis grade in treated AIH patients (18), and a non-invasive inflammatory score was proposed to discriminate patients with and without significant hepatic inflammation (19). The above mentioned score is easy to calculate but would be only applicable to patients without co-morbidities and would not account for patients with low inflammatory activity (20).
2. Objectives
The aim of the present study was to evaluate the performance and validity of TE, other non-invasive markers of liver fiibrosis, and the inflammatory score proposed by Gutkowski et al. in patients with biopsy-proven AIH against the golden standard of liver biopsy and to assess if one or more non-invasive markers could replace liver biopsy.
3. Methods
3.1. Patients
One hundred-three (103) patients were identified from their registration in the department of gastroenterology and hepatology at the University of Essen-Duisburg between 2008 and 2013. Fifty-three (53) of them fulfilled the inclusion criteria (vide infra) and included in this retrospective analysis. AIH was diagnosed according to the diagnostic criteria proposed by the international autoimmune hepatitis group and the patients included in the study fulfilled the criteria of probable or definite AIH (21).
Inclusion criteria were the presence of AIH and the performance of liver biopsy in addition to liver stiffness measurement. Every patient included in the study underwent liver biopsy within 6 months of their liver stiffness measurement, with a median of 4 (2-17) days. Exclusion criteria were the presence of ascites, diagnosis of hepatocellular carcinoma (HCC), severe obesity (BMI > 40 kg/m2), hepatitis B or C virus infection, metabolic liver diseases (ASH, NASH, Wilson’s disease, etc.) and overlap syndromes to primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC).
3.2. Histology
Ultrasound-guided percutaneous liver biopsy was performed on 19 (35.8%) patients and laparoscopic biopsy was performed on 34 (64.2%) patients as described before (22). Liver biopsy specimens were fixed in formalin and embedded in paraffin. All specimens were analysed independently by an experienced liver pathologist (H.B.) blinded to the study. Liver fibrosis and necro-inflammatory activity were evaluated according to the METAVIR scoring system (23). Fiibrosis was scored on a scale of 0-4 as follows: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis and few septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis. Inflammatory activity was graded as follows: A0 = none, A1 = minimal, A2 = mild, A3 = moderate, and finally, A4 = severe with A1 corresponding to 1 - 3 of modified HAI Score, A2 to 4 - 8, A3 to 9 - 12, and A4 to 13 - 18 (24). In the conversion of Ishak to METAVIR grading system, an Ishak 3 score corresponded to F2 (25). The length of each liver biopsy specimen and number of fragments were recorded and only those with a minimum length of 14 mm including at least 10 - 15 portal spaces were considered.
3.3. Laboratory Parameters
Blood values measured at our central laboratory are listed in Table 1. Following parameters were monitored: aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (ɣ-GT), glutamate dehydrogenase (GLDH), alkaline phosphatase (ALP), platelets, protein profile, total and direct bilirubin, cholesterol, cholinesterase, prothrombin-time, international normalised ratio (INR), partial thromboplastin-time (aPTT), leukocytes, haemoglobin, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides, C-reactive protein (CRP), and immunoglobulin G (IgG) were measured on the day or the day before TE performance.
| Variables | Mean ± SE |
|---|---|
| Male: 22 (41.5); Female: 31 (58.5) | |
| Age, y | 47.30 ± 2.39 |
| AST, UI/L | 418.09 ± 96.87 |
| ALT, UI/I | 606.42 ± 131.13 |
| ɣ-GT, UI/l | 248.92 ± 50.04 |
| Total bilirubin, mg/dL | 3.89 ± 0.8 |
| Direct bilirubin, mg/dL | 3.17 ± 0.78 |
| Alkaline phosphatase, U/I | 159.64 ± 16.47 |
| Cholinesterase, U/mL | 6.17 ± 0.42 |
| GLDH, U/I | 33.9 ± 7.56 |
| TPZ % | 86.17 ± 3.16 |
| INR | 1.13 ± 0.03 |
| aPTT (sec) | 28.43 ± 0.55 |
| Total protein, g/L | 7.42 ± 0.14 |
| Albumin, g/L | 3.91 ± 0.09 |
| Platelets, /nL | 208.79 ± 14.04 |
| Leukocytes, /nL | 6.37 ± 0.46 |
| Hemoglobin, g/dL | 13.35 ± 0.27 |
| Cholesterol, mg/dL | 209.17 ± 19.8 |
| LDL, mg/dL | 136.56 ± 20.64 |
| HDL, mg/dL | 38.40 ± 8.96 |
| Triglycerides, mg/dL | 126.17 ± 13.12 |
| CRP, mg/dL | 8.38±1.63 |
| Immunglobulin, G, g/L | 18.44 ± 1.52 |
| Fiibrosis stage: F1/F2/F3/F4, No. (%) | 9 (17) / 15 (28.25)/ 14 (26.5) / 15 (28.25) |
| Inflammatory activity: A0-1/A2/A3/A4, No. (%) | 21 (41.5) / 9 (17) / 9 (17) / 13 (24.5) |
Characteristics of the Study Populationa
3.4. Liver Stiffness Measurement
All patients were assessed for the following surrogate markers of liver fiibrosis: APRI (5), FIB-4 (6), FibroQuotient (FibroQ) (7), and AST/ALT ratio (8, 9). NAFLD fiibrosis score could be calculated in 41 patients (10). Inflammatory score and fiibrosis score by Gutkowski were calculated as described earlier (19). Liver stiffness measurement by transient elastography was performed using FibroScan (Siemens Healthcare Erlangen, Germany) on the right hepatic lobe, on an area at least 6 cm in thickness without major blood vessels. Only procedures with 10 validated measurements and a greater than 60 % success rate were considered as reliable. Median value of successful measurements was considered to be representative only if the interquartile range of all measurements was less than 25%. Results were expressed in kilopascal (kPa).
3.5. Statistical Analysis
Continuous data were expressed as mean ± standard error (SE) of the mean. Categorical data were described as frequencies of the subjects with a specific characteristic. Area under the receiver operating characteristic (AUROC) curves were plotted to define the best cut-off point to distinguish different stages of fiibrosis. Optimal cut-off values between fiibrosis stages were determined at the maximum sum of sensitivity and specificity. Bivariate Spearman’s rank correlation (rs) was used to analyze the correlation between models and degree of fiibrosis. Logistic regression analysis was performed to assess the relationship between fiibrosis stage and liver stiffness (LS) or other non-invasive markers. Pearson coefficient of correlation (r) was calculated next. Two-tailed p-values less than 0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA).
3.6. Compliance With Ethical Standards
Research involving human participants: The study was conducted in accordance with the ethical guidelines of the 1975 Helsinki Declaration, and the protocol was approved by the ethics and research committees of the University Clinic of Essen-Duisburg.
3.7. Informed Consent
Informed consent was waived due to the retrospective character of the study.
4. Results
4.1. Characteristics of the Study Population
Anthropomorphic characteristics and laboratory parameters are summarized in Table 1. Twenty seven patients had a BMI < 25 kg/m2, 21 patients were overweight, while 5 patients were obese. Most patients had AIH type I (n = 50, 94.3%) and three patients suffered from AIH type II (5.7%). The measured liver stiffness (LS) ranged from 3.2 to 75 kPa with a mean ± SE of 15.41 ± 1.93 kPa.
We found a statistically significant positive correlation between grade of fiibrosis according to the METAVIR scoring system and the transient elastography score (r = 0.531; P < 0.001), FIB-4 (r = 0.332; P = 0.016), NAFLD fiibrosis score (r = 0.731, P < 0.0001), and FibroQ-Index (r = 0.580; P < 0.001). However, we found no significant correlation between grade of fiibrosis and APRI (r = 0.163; P = 0.25), AST/ALT-ratio (r = 0.372; P = 0.07) or fiibrosis score by Gutkowski (r = 0.011; P = 0.938).
4.2. Liver Stiffness Measurements
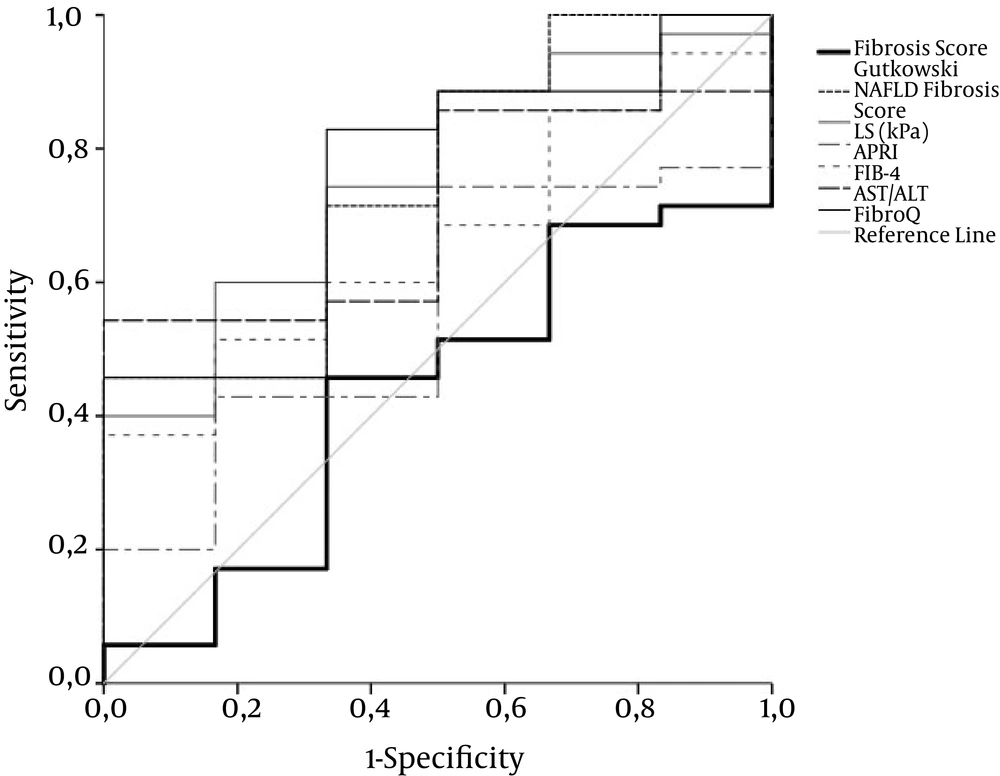
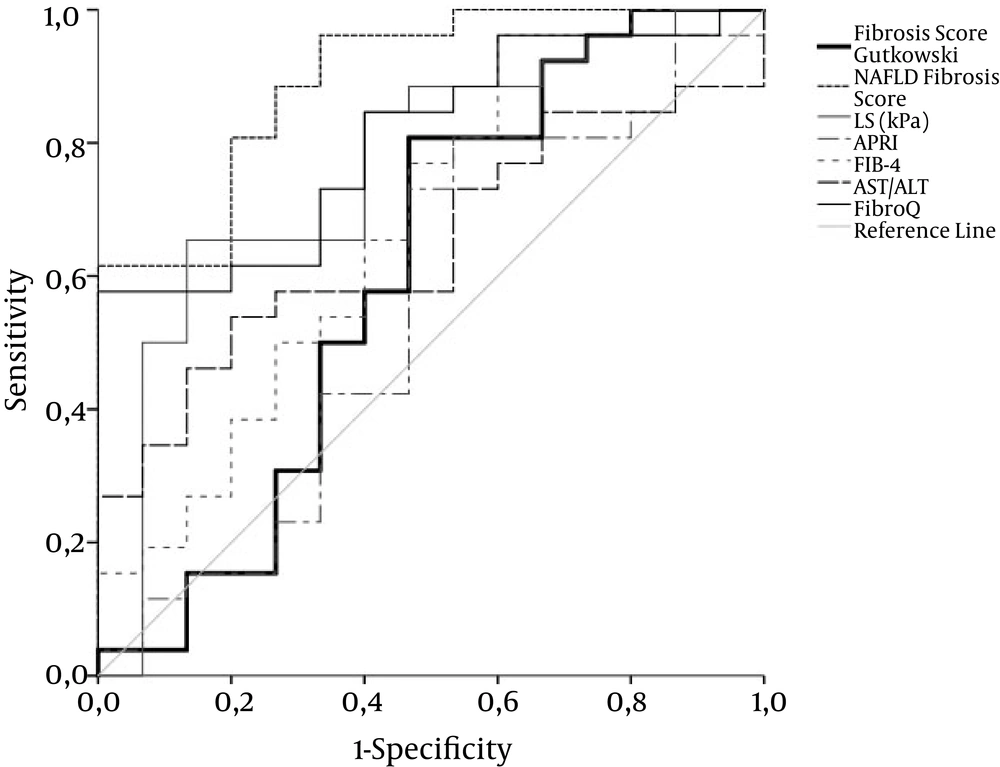
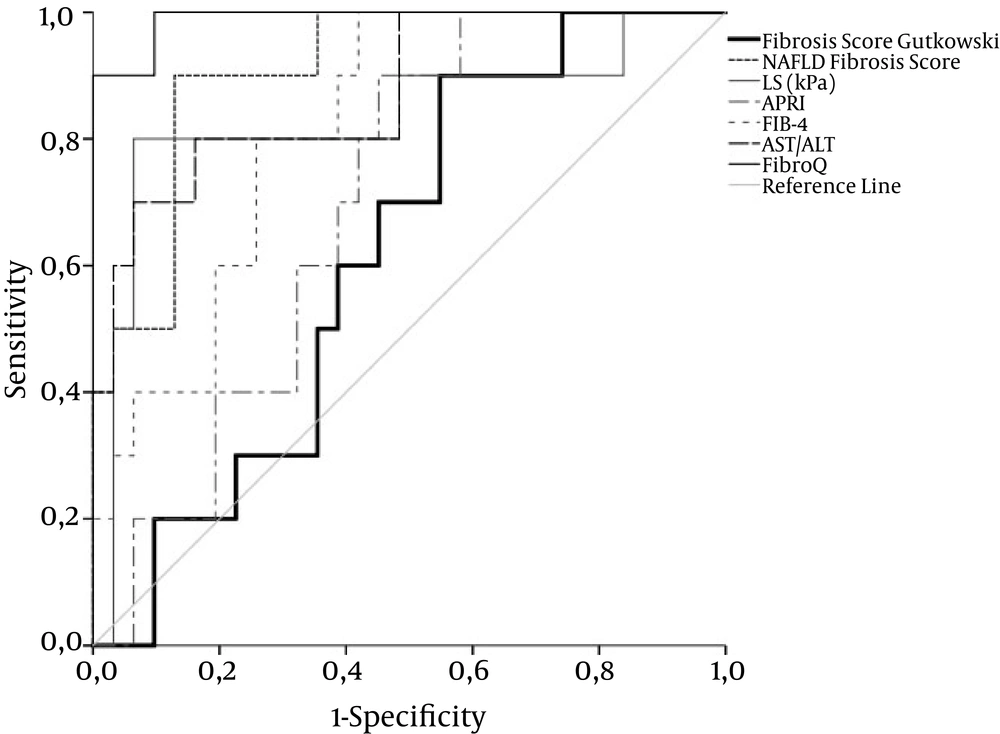
Figure 1 demonstrate the diagnostic performance (ROC curves) of liver stiffness (LS) by comparison with non-invasive markers of fiibrosis stages ≤ 1, ≤ 2, and = 4, respectively. Optimal cut-off value for liver stiffness was 10.05, 12.1, and 19 kPa for fiibrosis stages ≤ 1 versus all other stages of fiibrosis, fiibrosis stages ≤ 2, and fiibrosis stage 4, respectively. Areas under the curve (AUC), sensitivity, specificity, positive and negative likelihood ratios for LS are shown in Table 2. Furthermore, there was no correlation between LS and histological inflammation grade.
Only the ROC curve for TE exhibits acceptable overall performance to discriminate F1 from F2 - 4. Diagonal segments are produced by ties. Areas under the curve are presented in Table 2.
ROC curves for TE, Fibro Q, and NAFLD fiibrosis score exhibit acceptable overall performance to discriminate F1 - 2 from F3 - 4, with NAFLD fiibrosis score achieving the highest discriminatory performance. Diagonal segments are produced by ties. Areas under the curve are presented in Table 2.
ROC curves for TE, FIB-4, AST/ALT, Fibro Q, and NAFLD fiibrosis score exhibit acceptable overall performance to discriminate F1 - 3 from F4, with FibroQ achieving the highest discriminatory performance. Diagonal segments are produced by ties. Areas under the curve are presented in Table 2.
| Variable | AUC ± SE | Optimal Cut-Off | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | P |
|---|---|---|---|---|---|---|---|
| F1 versus F2 - 4 | |||||||
| LS, kPa | 0.779 ± 0.08 | 10.05 | 61.4 | 88.9 | 96.4 | 31.8 | 0.009 |
| APRI | 0.601 ± 0.09 | 1.45 | 59.1 | 67 | 89.7 | 25.1 | 0.343 |
| FIB-4 | 0.659 ± 0.08 | 3.2 | 47.7 | 88.9 | 95.5 | 26 | 0.136 |
| AST/ALT | 0.601 ± 0.09 | 0.718 | 52.3 | 77.8 | 92 | 25 | 0.343 |
| FibroQ | 0.684 ± 0.09 | 1.34 | 72.7 | 66.7 | 91.5 | 33.7 | 0.084 |
| NAFLD fiibrosis score | 0.752 ± 0.1 | -2.6 | 71.4 | 66.7 | 91.3 | 32.1 | 0.051 |
| Fiibrosis score Gutkowski | 0.433 ± 0.12 | 2.96 | 45.7 | 66.7 | 87.2 | 20.3 | 0.606 |
| F1-2 versus F3 - 4 | |||||||
| LS, kPa | 0.739 ± 0.07 | 12.1 | 58.6 | 83.3 | 80.9 | 62.4 | 0.003 |
| APRI | 0.527 ± 0.08 | 1.24 | 69 | 50 | 62.8 | 59.9 | 0.734 |
| FIB-4 | 0.614 ± 0.08 | 1.93 | 75.9 | 50 | 65 | 35 | 0.158 |
| AST/ALT | 0.655 ± 0.08 | 0.795 | 48.3 | 75 | 70.1 | 54.1 | 0.054 |
| FibroQ | 0.773 ± 0.06 | 2.695 | 58.6 | 91.7 | 90 | 64.7 | 0.001 |
| NAFLD fiibrosis score | 0.895 ± 0.05 | -3.23 | 92.3 | 66.7 | 77.3 | 87.3 | < 0.0001 |
| Fiibrosis score Gutkowski | 0.605 ± 0.1 | 2.599 | 80.8 | 53.3 | 68.1 | 70.7 | 0.267 |
| F1-3 versus F4 | |||||||
| LS, kPa | 0.842 ± 0.09 | 19 | 81.8 | 92.9 | 75.7 | 95.1 | 0.001 |
| APRI | 0.665 ± 0.08 | 1.848 | 81.8 | 57.1 | 33.6 | 92.3 | 0.096 |
| FIB-4 | 0.766 ± 0.08 | 2.61 | 90.9 | 59.5 | 37.7 | 96 | 0.007 |
| AST/ALT | 0.799 ± 0.08 | 0.939 | 63.6 | 88.1 | 58.6 | 90.2 | 0.002 |
| FibroQ | 0.916 ± 0.06 | 3.999 | 81.8 | 92.9 | 75.7 | 95.1 | < 0.0001 |
| NAFLD fiibrosis score | 0.910 ± 0.05 | -1.095 | 90 | 87.1 | 64.8 | 97 | < 0.0001 |
| Fiibrosis score Gutkowski | 0.619 ± 0.09 | 2.66 | 90 | 45.2 | 30.3 | 94.4 | 0.261 |
Diagnostic Performance of LS, APRI, FIB-4, AST/ALT, FibroQ, NAFLD Fiibrosis Score, and Fiibrosis Score by Gutkowski for Determination of Fiibrosis Stage
4.2.1. Which of the non-invasive markers of liver fiibrosis performed better for fiibrosis stages ≤ 1, ≤ 2, and = 4?
The overall performance of NAFLD fiibrosis score was better than that of other laboratory markers. The performance of FibroQ was the best in differentiating severe fiibrosis and cirrhosis from mild to moderate fiibrosis, but it was inadequate to differentiate mild from more advanced fiibrosis stages. APRI and fiibrosis score by Gutkowski had the worst performance. All markers with the exception of APRI and fiibrosis score by Gutkowski demonstrated an acceptable sensitivity and specificity in differentiating fiibrosis stage 4. Areas under the curve (AUC), sensitivity, specificity, positive and negative likelihood ratios for the aforementioned parameters are shown in Table 2.
In the logistic regression model with univariate analysis of the parameters related to advanced fiibrosis (i.e. histological stages III and IV), the variables significantly associated with the extent of fiibrosis were AST/ALT (P = 0.024, Exp (B) 11.226; 95 % CI for Exp (B) 1.367 - 92.217), NAFLD fiibrosis score (P = 0.002, Exp (B) 3.932; 95 % CI for Exp (B) 1.687 - 9.167), and FibroQ (P = 0.007, Exp (B) 2.002; 95 % CI for Exp (B) 1.206 - 3.323). In the logistic regression model with univariate analysis of the parameters related to fiibrosis stage 4, the variables significantly associated with the extent of fiibrosis were LS (P = 0.003, Exp (B) 1.098; 95 % CI for Exp (B) 1.034 - 1.167), FIB-4 (P = 0.01, Exp (B) 1.305; 95 % CI for Exp (B) 1.065 - 1.1599), AST/ALT (P = 0.003, Exp (B) 31.474; 95 % CI for Exp (B) 3.209 - 308.674), NAFLD fiibrosis score (P = 0.03, Exp (B) 4.003; 95 % CI for Exp (B) 1.602 - 9.999), and FibroQ (P = 0.003, Exp (B) 2.945; 95 % CI for Exp (B) 1.461 - 5.936).
4.2.2. Did the inflammatory score by Gutkowski correlate with the grade of histological inflammation?
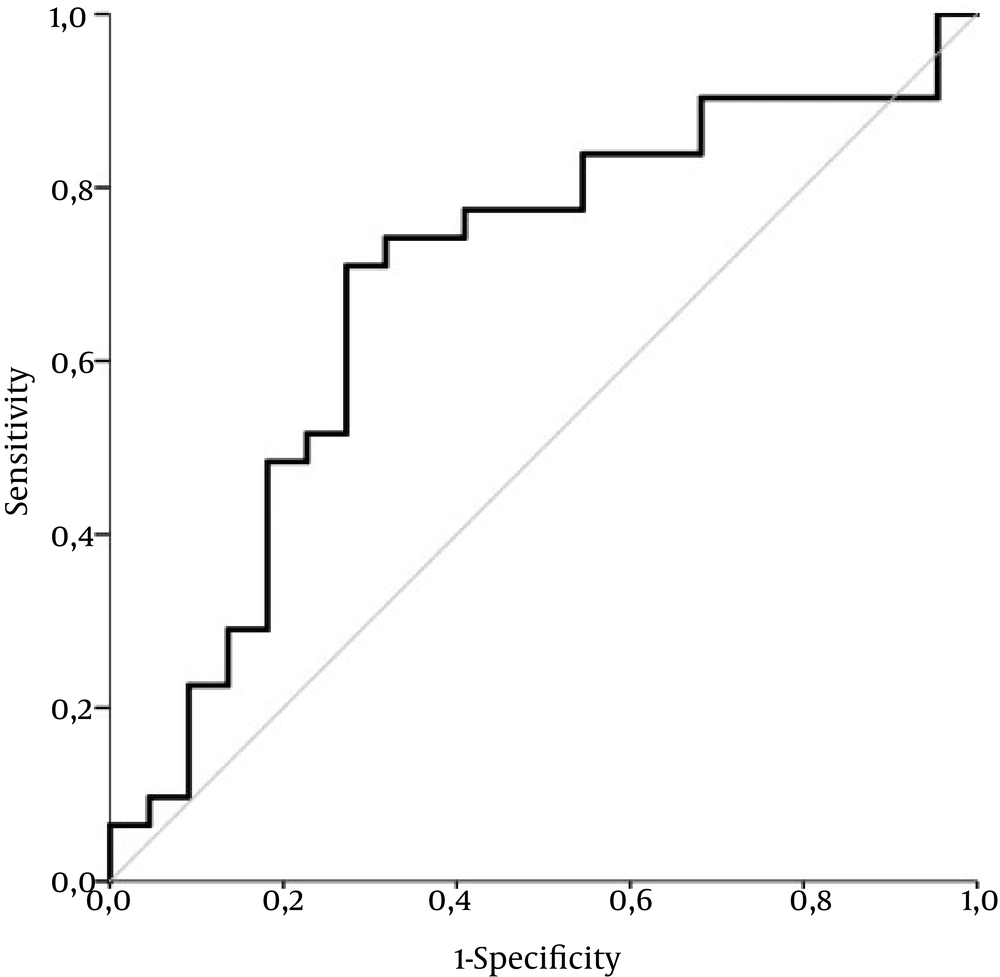
We observed a statistically significant positive correlation between histologically evaluated inflammation grade and the inflammatory score by Gutkowski with r = 0.409 and P = 0.002. Univariate logistic analysis revealed, however, no statistically significant association with P = 0.069, Exp (B) 1.146; 95 % CI for Exp (B) 0.990 - 1.327. The performance of inflammatory score by Gutkowski was moderately acceptable with an AUC of 0.688 ± 0.08 (P = 0.021) in differentiating minimal and mild (A1 - 2) from moderate to severe inflammation grade (A3 - 4) (Figure 4).
4.2.3. Did immunosuppressive therapy influence the predictive ability of non-invasive fiibrosis markers?
We separated our collective into two groups: 18 patients, who received immunosuppressive therapy for at least one year, and 35 therapy-naive patients. Therapy-naive patients were more likely to have moderate or severe inflammation compared to treated patients (19 vs. 3, P= 0.01). ROC curves were plotted for LS, APRI, FIB-4, AST/ALT, and FibroQ. Results are presented in Table 3. In untreated patients, TE could differentiate F1 from F2 - 4 and F1 - 2 from F3 - 4 better than the other markers, while FibroQ had a superior performance in differentiating patients with cirrhosis (F4 from F1 - 3). In treated patients, only TE had an adequate performance in differentiating F1 - 2 from F3 - 4 patients, while FibroQ demonstrated an inferior performance.
| F1 Versus F2 - 4 | F1-2 Versus F3 - F4 | F1-3 Versus F4 | ||||
|---|---|---|---|---|---|---|
| Therapy Naive | Treated | Therapy Naive | Treated | Therapy Naive | Treated | |
| LS, kPa | 0.809 ± 0.09a | 0.813 ± 0.1 | 0.822 ± 0.08a | 0.815 ± 0.1a | 0.966 ± 0.034b | 0.754 ± 0.15 |
| APRI | 0.689 ± 0.1 | 0.5 ± 0.15 | 0.556 ± 0.1 | 0.723 ± 0.12 | 0.615 ± 0.1 | 0.815 ± 0.12a |
| FIB-4 | 0.77 ± 0.08a | 0.344 ± 0.15 | 0.655 ± 0.09 | 0.708 ± 0.15 | 0.782 ± 0.094a | 0.831 ± 0.14a |
| AST/ALT | 0.633 ± 0.1 | 0.375 ± 0.14 | 0.671 ± 0.1 | 0.554 ± 0.14 | 0.908 ± 0.071a | 0.615 ± 0.15 |
| FibroQ | 0.765 ± 0.09a | 0.406 ± 0.12 | 0.78 ± 0.08a | 0.738 ± 0.13 | 0.977 ± 0.03b | 0.846 ± 0.14a |
Diagnostic Performance of LS, APRI, FIB-4, AST/ALT, and FibroQ for Determination of Fiibrosis Stage in Untreated and Treated Patients, Expressed as Area Under the Curve ± Standard Error (AUC ± SE) in ROC Analysis
5. Discussion
In the present study, we evaluated the performance and validity of transient elastography and other non-invasive markers of liver fiibrosis in patients with biopsy-proven autoimmune hepatitis. Both TE and most of the non-invasive markers correlated positively with various grades of fiibrosis, as expected. However, the diagnostic performance of TE was not as precise as expected from previous studies (26-28).
The diagnostic performance for fiibrosis stage I versus stage II-IV was better for TE in comparison with other non-invasive markers with ROC of 0.779 ± 0.08 but still with relatively low sensitivity. The diagnostic performance of TE did not improve for fiibrosis stages 1 - 2 versus 3 - 4 with 0.739 ± 0.07, and was worse than the performance of FibroQ or NAFLD fiibrosis score but still better than that of other non-invasive markers. TE performed better in differentiating stage IV fiibrosis from all other stages with 0.842 ± 0.09 but still did not outperform FibroQ. The inflammatory score, used to predict inflammatory activity, performed moderately good with an AUROC of 0.688.
These results demonstrated that TE is an effective tool to differentiate fiibrosis stage IV from other stages of liver fiibrosis but not necessarily better than other parameters such as FibroQ or NAFLD fiibrosis score - which are easier to obtain. However, there is a slight advantage in comparison with other non-invasive markers in differentiating fiibrosis stage I from other fiibrosis stages but both its sensitivity and specificity in our collective were not impressive. In addition, our results revealed a lower diagnostic value for TE in AIH in comparison with studies evaluating TE and non-invasive markers of liver fiibrosis in patients with other chronic liver diseases (26-28). A better diagnostic performance of TE in comparison with other non-invasive markers was reported in a study of HCV transplant recipients by Kamphues and colleagues (29) as well as in a study of our center with transplant recipients with and without HCV (30). Much lower optimal cut-off points were set in the above mentioned study when differentiating fiibrosis grade in transplant recipients, and TE diagnostic performance ranged from 0.85 to 0.99 - a scoring better than scoring in our collective. A recent study by Xu et al. (17), testing the diagnosing accuracy of LS, APRI, and FIB-4 in 100 AIH patients, demonstrated similar diagnostic performances for these markers like in our study. In their study, TE outperformed the other markers; however, FibroQ was not calculated. A study of Floreani et al. and Corpecot et al. (31, 32) in PBC patients indicated a good diagnostic performance of TE, especially for fiibrosis stages ≤ 2 and = 4. In all three studies the optimal cut-off points were set lower in comparison with our study. Concerning the laboratory non-invasive markers of liver fiibrosis, the diagnostic performance of FIB-4 (0.614 vs. 0.55) and AST/ALT (0.655 vs. 0.57) was better in our study in comparison with the study of Abdollahi and colleagues, while the diagnostic performance of APRI was similarly poor. The above mentioned study did not include FibroQ, NAFLD fiibrosis score or TE. In addition, a study conducted on 39 AIH patients suggested AST/ALT-ratio as a good predictor of advanced fiibrosis (15).
A recent study on AIH patients suggested that TE performs better in AIH patients treated more than 6 months compared to patients treated less than 3 months or untreated to differentiate patients with cirrhosis (18). We could not demonstrate a similar result, however, the number of treated patients was small (n = 18). FibroQ outperformed TE in predicting cirrhosis in both treated and untreated patients.
Limitations of our study are its small sample size and retrospective character. Most non-invasive markers including TE correlated positively with the grade of fiibrosis. Compared to other studies, transient elastography did not perform as well as expected and according to our results, it could not replace liver biopsy in differentiating fiibrosis grade in patients with AIH. The results of laboratory non-invasive fiibrosis markers (APRI, AST/ALT, and FIB-4) were slightly better and comparable to those from other studies. Although FibroQ and NAFLD score performed better in patients with severe fiibrosis, they showed a slightly worse performance than TE in patients with mild fiibrosis. TE, Fibro Q, and NAFLD score performed well in differentiating cirrhosis from less advanced forms of fiibrosis with a ROC > 0.8, while only NAFLD fiibrosis score performed well in differentiating F1 - 2 from F3 - 4 with a ROC of 0.895. The performance of TE in patients with cirrhosis was acceptable but not better than that of non-invasive laboratory markers which are easier to obtain. All non-invasive fiibrosis markers performed sub-optimally in differentiating mild from more advanced stages of fiibrosis. Non-invasive fiibrosis markers seem to have a limited diagnostic potential in AIH but may be of value for the follow-up of patients with AIH, a hypothesis that could be evaluated in future studies.
In conclusion, non-invasive markers of liver fiibrosis could differentiate reliably cirrhosis from less advanced stages of fiibrosis in our collective of patients with AIH, but their diagnostic performance was inadequate to differentiate mild from more advanced stages of fiibrosis and thus could not replace liver biopsy as the golden standard for fiibrosis staging in patients with AIH.