1. Context
Hepatitis C virus (HCV) infection is a major public health issue worldwide. This infection is one of the major causes of chronic hepatitis, with the risk of progression to cirrhosis and development of Hepatocellular Carcinoma (HCC). The majority of infected patients do not clear the acute infection and progress to chronic infection (1). Unfortunately, most of the infected patients are not aware of their disease (2).
1.1. Epidemiology and Burden of Hepatitis C
Based on the data reported by the world health organization (WHO), the global prevalence of HCV infection is estimated as 2.2% or 130 million, with more than one million new cases reported annually. Furthermore, after hepatitis B Virus (HBV), HCV is the second cause of cirrhosis and HCC in the world, while it is the first etiology of advanced liver diseases in some regions (3-5). The global epidemiology of HCV varies substantially across the world (6). In Africa, HCV prevalence is 5.3%, equating 31.9 million infected patients. In the Eastern Mediterranean Region (EMRO), HCV prevalence is 4.6%, equating 21.3 million (7). Iran has the lowest prevalence for HCV infection in the Middle East. It has been estimated that the prevalence of HCV in the Iranian general population is less than 0.5%, equating 186500 individuals (8). The main populations at risk of HCV infection in Iran include intravenous drug users (IDUs) followed by people with hemophilia, thalassemia, and patients on hemodialysis (9-13). Countries such as Pakistan and Azerbaijan with high prevalence of HCV infection are neighbors of Iran. Unfortunately, the security problems and wars in the Middle East has damaged the health infrastructure of several countries and there are concerns of worsening situation of HCV infection in the region in the near future (14).
Vaccination against HBV infection has decreased the burden of HBV in the world and in the region (15, 16). It seems that HCV will emerge as the leading cause of viral hepatitis-related advanced liver diseases and death in the near future in Middle Eastern countries, including Iran. Increasing burden of HCV-related advanced liver diseases and death is expected in the future in Iran (8). Strategies for increasing the rate of HCV diagnosis and treatment with high efficacy antiviral agents are required to reverse the rising tide of HCV burden. Young HCV-infected population in Iran provides an opportunity for timely interventions to limit the burden of HCV disease in the future.
1.2. Preventive Strategies
There is no vaccine for HCV prevention yet and integrated preventive measures on the main risk factors are crucial to save the community. The incidence of HCV infection should be reduced by providing safe blood transfusion and medical procedures in hospitals and out-patient clinics, increasing people awareness and public education regarding the risks of exposure such as unsafe tattooing and unsafe sexual contacts and finally implementation of harm reduction for IDUs. The majority of young people who acquire HCV from injecting drug use develop chronic infection. Preventing transmission of HCV among young IDUs is critically important, yet it is a difficult task. Most youth are unaware of the risk of acquiring HCV from drug use. Needle exchange programs can reduce the risk of infection (17). Hepatitis C treatment programs and intensive community-based education programs will remain the mainstays of HCV prevention (18). Today, it seems that the best strategy for HCV prevention in the community is increasing case finding and therapy with the ultimate goal of stopping the vicious cycle in the community.
1.3. Screening to Identify People with Hepatitis C Virus Infection
Fortunately, after screening of blood donors for HCV in Iran, the burden of HCV infection decreased significantly in hemophilia, thalassemia and patients on hemodialysis. Unfortunately, injecting illicit drugs still continues to be a major source of infection in Iran. Given that more than 60% of prisoners in Iran are sentenced due to drug-related crimes, HCV screening in the prisons are required. Strategies to promote diagnosis, screening, and treatment should be targeted to high-risk groups rather than the general population. Beside the high prevalence of HCV infection in IDUs, this group also constitutes to be a potential reservoir for HCV in the community. It is important to implement an annual HCV screening in high-risk groups, given the ongoing exposure to HCV infection in these populations.
Based on the previous epidemiological studies in Iran (19), we recommend screening of HCV through anti-HCV antibody (HCV Ab) testing for the following groups: 1, Individuals with a history of blood or blood product transfusion before 1995 (time of blood screening for HCV in Iran); 2, Individuals with a history of war wounds; 3, Individuals with a history of injecting illicit drugs; 4, Individuals with history of imprisonment; 5, Patients with hemophilia or thalassemia and those on hemodialysis; 6, Patients receiving organ transplantation; 7, Individuals with high-risk sexual behavior, including sex workers and individuals with multiple sex partners; 8, Individuals with a history of tattooing or traditional phlebotomy such as Hejamat; and 9, Children born to HCV-infected mothers.
1.4. Genetics and Molecular Testing in Hepatitis C Virus Infection
Genetic variability is a distinctive feature of HCV. Hepatitis C viral sequences are currently classified to seven different genotypes (20). There is a certain geographical distribution of HCV genotypes. HCV genotype 1 (HCV-G1) is the most common genotype in the United States and Europe (6). The genotype distribution of HCV has diverse patterns in EMRO countries. Furthermore, HCV-G1 and -G3 are predominant in Iran and Pakistan, while HCV-G4 and -G1 are the most common genotypes in Arab countries in the Middle East and North Africa (6).
Assessment of HCV genotypes is very useful for prediction of response to antiviral therapy with Pegylated-Interferon (PegIFN) and Ribavirin (RBV), which was higher in HCV-G2 and -G3 than HCV-G1 and -G4 (21). Today, with new antiviral therapies, the impact of HCV genotype on response to treatment is less than before. The impact of polymorphisms near IFNL3 gene on Sustained Virological Response (SVR) in PegIFN-based therapy was very interesting (22). The European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) included IFNL3 testing in their guidelines, yet today with the Direct-Acting Antiviral (DAA) regimens, there is no need for IFNL3 testing in clinical management of HCV infection.
Naturally occurring substitutions, which confer the decrease in the susceptibility to DAAs are called Resistance-Associated Variants (RAVs). The RAVs can be observed in a proportion of patients by molecular methods (Sanger sequencing and next-generation sequencing) prior to treatment and can be selected through the pressure of the antiviral medications (23). As a result, most of the patients with treatment failure harbor the viral isolates with RAVs. Sofosbuvir (SOF) is the only approved NS5B nucleotide analog with high barrier to resistance and the NS5B Ser282Thr RAV is rarely observed in SOF-containing clinical trials neither in baseline samples nor in patients experiencing treatment failure (23). The NS5A RAVs are detected in 10 to 30% of patients at baseline and these RAVs can be observed in most patients with viral relapse. Based on clinical trials, patients with baseline NS5A RAVs will have lower chance of treatment response than patients without baseline NS5A RAVs (24, 25). It seems that assessment of baseline NS5A RAVs can have a role in optimization of treatment with NS5A inhibitors including Ledipasvir (LDV) and Daclatasvir (DCV).
1.5. Assessing the Degree of Liver Fibrosis and Cirrhosis
Accurate assessment of liver fibrosis and cirrhosis is essential for predicting prognosis and for planning treatment duration and adding RBV to the standard therapy of patients with chronic HCV infection (26, 27). For many years, percutaneous liver biopsy has been considered as the gold standard for assessing hepatic fibrosis. However, new non-invasive methods such as elastography measure the mean stiffness of hepatic tissue with hepatic rigidity being considered a marker of progressive fibrosis. The Fibroscan can help in exclusion of persistence of liver cirrhosis in HCV-infected patients.
2. Evidence Acquisition
PubMed, Scopus and Web of Science were searched systematically with appropriate combination of the following keywords: “Hepatitis C”, “HCV”, “Treatment”, “DAA”, “Direct-acting antiviral”, “Sofosbuvir”, “Ledipasvir”, “Daclatasvir”, “Simeprevir”, “Ombitasvir”, “Paritaprevir”, “Dasabuvir”, “Elbasvir”, “Grazoprevir”, “Velpatasvir”, “NS5A Inhibitor”, “NS5B Inhibitor”, “NS3 Inhibitor” and “Resistance”. Relevant articles were included after screening of the title and abstract by Seyed Moayed Alavian, Heidar Sharafi, Mohammad Saeid Rezaee-Zavareh, Bita Behnava and Khashayar Hesamizadeh. Included articles were reviewed to collect data on effectiveness and safety of various HCV treatment regimens. Based on the affordability and availability of HCV treatment regimens and the consensus of Iran Hepatitis Scientific Board (IHSB), the recommendations were finalized by the third national consensus on management of Hepatitis C in Iran held on 22nd of July 2016.
3. Results
3.1. Definitions
The goal of treatment of chronic HCV infection is the clearance of the virus from plasma. This can be defined as the absence of detectable HCV RNA, 12 weeks (SVR12) or 24 weeks (SVR24) after termination of treatment. SVR12 and SVR24 are generally accepted as adequate evidence of cure by regulatory bodies, given that the late relapse is very rare in patients achieving SVR12 (28).
3.2. Pegylated-Interferon and Ribavirin Treatment of Hepatitis C Virus Infection
Before year 2011, combination of weekly PegIFNα and RBV in a 24- or 48-week course, was the standard of care for chronic HCV (29), which was associated with many side effects, including anemia, depression, decompensation and thrombocytopenia. The rate of SVR was affected by the baseline HCV RNA level and HCV genotypes; 70% - 90% for HCV-G2 and -G3, and almost 50% for HCV-G1 and -G4 (30). In addition, SVR was affected by polymorphisms near the IFNL3 gene (22). Patients with the rs12979860 CC genotype were two or three times more likely to respond to HCV clearance with PegIFNα plus RBV dual therapy than those with the CT or TT genotypes (31).
The studies on treatment of HCV with PegIFNα and RBV showed excellent response to this combination therapy in Iranian patients either in patients with HCV-G1 or -G3 infection (32).
3.3. Treatment of Hepatitis C Virus Infection with DAAs
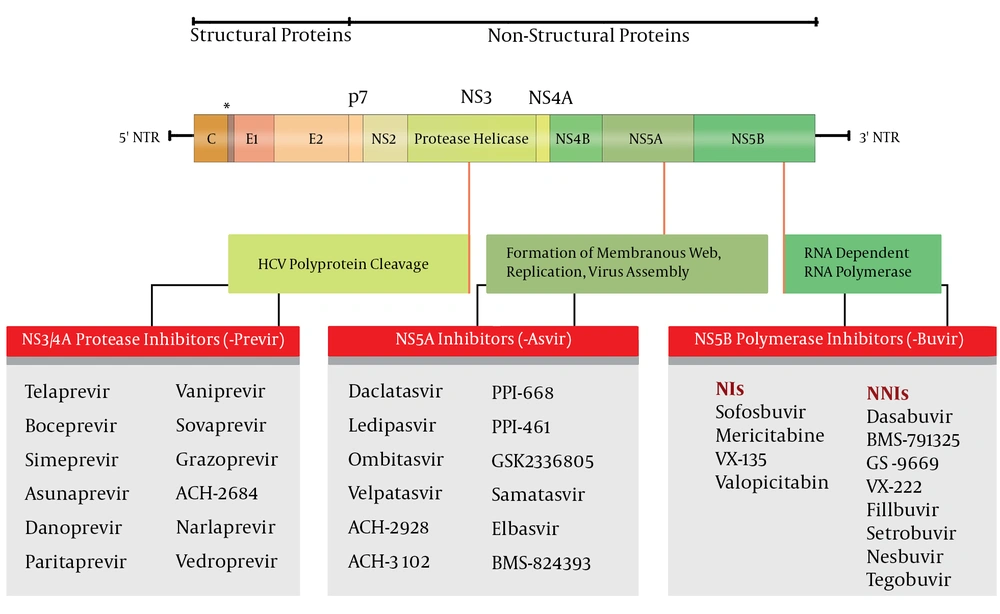
Treatment of chronic HCV has evolved over the years (Figure 1). In 2011, protease inhibitors, the first generation of DAAs, emerged as the third component of the standard of care given the increased SVR by triple therapy. The protease inhibitors that were recommended for use in clinical practice, Telaprevir (TVR) and Boceprevir (BOC), were effective in elimination of HCV through forming a reversible but covalent complex with the HCV NS3/4A serine protease catalytic site. The utilization of TVR and BOC in clinical practice was limited by their unfavorable pharmacokinetic profile, the significant number of drug-drug interactions, adverse events such as severe skin rashes/pruritus, anemia, the risk of fatal complications in patients with advanced liver disease, and finally restriction to patients with HCV-G1 infection (33).
In 2013, Sofosbuvir, a new DAA, was introduced for treatment of HCV infection. SOF-containing regimens had a shorter duration of therapy, with fewer side effects in comparison with protease inhibitor-based triple therapy. Different trials of SOF in treatment-naive patients with HCV-G1 through -G6 showed that patients with HCV-G1 infection had higher virologic response than the response rates in PegIFN and RBV combination therapy and first generation protease inhibitor-based therapies. For patients with HCV-G2 and -G3, efficacy was similar between an IFN-free SOF regimen and a PegIFN and RBV regimen (34). Traditional predictors of treatment response, such as polymorphisms near IFNL3, baseline HCV RNA level, and early response do not seem to affect response rates in IFN-free regimens (35).
3.3.1. Sofosbuvir
Sofosbuvir is a potent nucleotide analogue of the HCV NS5B polymerase inhibitor that is administered orally. Sofosbuvir with a high barrier to resistance without any virologic breakthrough has a potent antiviral activity against all genotypes of HCV, although it might be less efficient against HCV-G3.
Many studies evaluated the efficacy of SOF in chronic HCV. The first study was ELECTRON that evaluated the safety and efficacy of SOF as a backbone of combination antiviral therapy in patients with chronic HCV-G1, -G2 and -G3 infections, including both treatment-naive and treatment-experienced patients (36). For patients with HCV-G1 infection, 12-week SOF plus RBV provided SVR12 in 84% of treatment-naive, and only 10% in treatment-experienced patients. Among patients with HCV-G2/3, receiving SOF mono-therapy, SVR was 60% (36). This study showed that SOF and RBV might be the backbone for treatment of HCV patients, yet it needs to be in combination with other antiviral agents. The NEUTRINO trial was a study of SOF plus PegIFN/RBV (37), the FISSION trial was a study of SOF plus RBV in patients with HCV-G2 or -G3 infection (37), and the POSITRON trial was a study of SOF and RBV in a treatment-experienced group.
3.3.2. Ledipasvir/Sofosbuvir
Ledipasvir is an HCV NS5A inhibitor with antiviral activity against HCV GT-1 and -G4 (38). Ledipasvir was used in combination with SOF in clinical studies, including ION-1 (24), ION-2 (25), and ION-3 (39). In naive, treatment-experienced, cirrhotic or non-cirrhotic patients, fixed-dose combination of LDV and SOF, with or without RBV for 12 or 24 weeks showed very high efficacy, and SVR was not less than 95% in patients with HCV-G1 infection. In most patients with relapse, resistance assessment showed the NS5A RAVs. Moreover, some of the cases with treatment failure also had HCV NS5A RAVs at baseline (24), yet at the time of relapse most of the patients had NS5A RAVs. In HCV GT-1a patients with relapse, the L31M variant and in HCV GT-1b, the Y93H variant was seen at the time of relapse. It seems that history of treatment and polymorphisms near IFNL3 did not affect the SVR but in patients with cirrhosis, using RBV increased SVR (40). Serious side effects were rare in these patients and rate of discontinuation due to adverse effects were very low. The most common side effects were fatigue, headache and nausea (24). In the SIRIUS study (41), previous non-responders with HCV-G1 and compensated cirrhosis treated with LDV/SOF and RBV for 12 weeks and had actual SVR very well (42).
3.3.3. Simeprevir
Simeprevir (SMV) is an orally-administered, reversible, specific and potent NS3/4A protease inhibitor for use in combined treatment regimens against chronic HCV infection (43). Simeprevir has a potent antiviral activity against all HCV genotypes, except HCV-G3. It has been approved in combination with other components such as PegIFN/RBV or SOF in HCV-G1 and -G4 in the United States and European countries (28). The main difficulty in treatment with SMV in clinical practice is the presence of NS3 Q80K polymorphism that reduces susceptibility to treatment, and occurs in approximately 20% of HCV-G1a (33). Similar to the other protease inhibitors, treatment with SMV is associated with emerging NS3 RAVs in clinical practice.
3.3.4. Daclatasvir
Daclatasvir is a pan-genotypic inhibitor of the NS5A protein of HCV, which seems to be promising for clinical practice. It has good antiviral potency against multiple HCV GTs, yet low barrier to resistance, which can be resolved by adding PegIFN and/or other DAAs to the regimens. The Food and Drug Administration (FDA) approved the use of DCV in combination with SOF, first for HCV-G3 (July 2015) and then for HCV-G1 (February 2016) (44). However, it had been approved in European Commission and European Medicines Agency across HCV-G1, -G2, -G3 and -G4 in 2014 (45).
Daclatasvir is generally safe and well tolerated with mild side effects. The efficacy and safety of DCV-containing regimens were demonstrated in the ALLY-1 and ALLY-2 phase 3 clinical trials. Finally, the combination of SOF and DCV, once daily with or without RBV, in patients with chronic HCV-G1, -G2, or -G3 infection resulted in SVR rates of up to 89-100% in treatment-experienced or -naive patients, patients with liver transplantation and HIV/HCV coinfected patients (46-49). The experience with the Iranian fixed-dose combination of DCV/SOF (Sovodak) indicated an SVR rate of 98% in both HCV-G1 and -G3 (Presented in the 3rd national consensus on management of Hepatitis C in Iran, Tehran, 22nd of July 2016). Daclatasvir has demonstrated an excellent safety profile when combined with SOF, including patients with decompensated liver disease, post-liver transplantation and HIV/HCV coinfection (46). Daclatasvir has a high profile of cross-resistance with LDV, and M28T, Q30R, L31M/V and Y93H/N RAVs are associated with resistance to DCV at baseline (23).
The evidence in HCV G4 is from DCV plus PegIFN and RBV with SVR12 rate of 82% in previously untreated patients. Serious adverse events associated with DCV were low (50).
3.3.5. Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir
Combination of four antiviral agents, Ombitasvir (OBV), an HCV NS5A inhibitor, Paritaprevir (PTV), an HCV NS3/4A protease inhibitor, Ritonavir (r), a CYP3A inhibitor, and Dasabuvir (DSV), an HCV nonnucleoside NS5B polymerase inhibitor, may be administered with or without RBV (28, 51). This combination is primarily excreted in the feces, although minimal elimination into the urine does occur. In patients with impaired renal function, exposure to PTV-r and DSV increases, however, the difference is not expected to be clinically relevant, and no dosage adjustment is required for patients with mild, moderate or severe renal impairment. Paritaprevir is potentially hepatotoxic. Cases of liver failure leading to liver transplantation or death have been attributed to the OBV plus PTV-r combination, administered with or without DSV. This combination carries a high-risk of pharmacokinetic interactions, making it difficult to use.
3.3.6. Grazoprevir/Elbasvir
Grazoprevir (GZR) (an NS3/4A protease inhibitor) and Elbasvir (EBR) (an NS5A inhibitor) are being developed for HCV-G1 and -4. The combination is administered as a once-daily, single-tablet regimen, with or without RBV. The two medications are active against multiple genotypes of HCV. This combination has no excretion through the kidneys, so these drugs can be used without dose adjustment in patients with renal failure. In phase 2 and 3 studies, GZR/EBR for 12 weeks, resulted in actual SVR rates among treatment-naive and treatment-experienced patients, who had cirrhosis and were infected with HCV-G1b. For treatment-experienced patients with HCV-G1a and -G4, a 16- to 18-week regimen with or without RBV was highly effective. The GZR/EBR combination regimen was generally well tolerated among patients with cirrhosis as well. The FDA approved the combination of GZR/EBR for HCV-G1 and -G4, in January 2016. Recently, in a study among treatment-naive patients with HCV-G1 infection treated by GZR/RBV, SVR12 after 12 weeks of treatment was only 58% while it was 90% after 24 weeks of treatment (52). The patients with relapse and breakthrough had wild-type virus before therapy, but they developed Y56H, V55A, A156T and D168A/N RAVs.
3.3.7. Velpatasvir/Sofosbuvir
Velpatasvir (VEL) is a new pan-genotypic HCV NS5A inhibitor with antiviral activity against all HCV-G1 through -G6. In July 2016, combination of VEL/SOF was approved as the first once-daily pan-genotypic single tablet regimen for the treatment of adults with all genotypes of HCV infection. This new combination therapy was approved, representing an important step towards elimination of HCV infection. The efficacy, safety and tolerability of VEL/SOF were supported by data from four phase 3 clinical studies, ASTRAL-1 (53), ASTRAL-2, ASTRAL-3 (54) and ASTRAL-4 (55).
The SVR rates among patients receiving 12 weeks of VEL/SOF was 95% - 99% in treatment-naive and -experienced patients (54, 56, 57) and 94% in decompensated cirrhosis patients, who received 12 weeks of VEL/SOF with RBV (55).
3.4. Management of Hepatitis C Infection
The future of HCV antiviral therapy will be IFN-free regimens with high-resistance barrier, curing nearly 100% of the infected patients. Keeping in mind that the ultimate HCV treatment is available or will be available in the near future, there is great interest to eliminate HCV infection globally until 2030 (58). Treatment recommendations in various genotypes and in patients with other co-morbidities have been discussed in the following sections. Availability of affordable DAAs with high insurance coverage for HCV treatment and testing are key to insure utilization of the recommendations.
We will explain here the affordable strategies with acceptable prices for use in clinical practices to improve our strategies for greater coverage of HCV-infected patients in treatment scenario. We tried to simplify and summarize the protocols for therapy in HCV-infected patients.
3.4.1. Variables in Decision to Treat
In PegIFN and RBV-based therapy, some features of HCV such as HCV genotype, and host factors such as polymorphisms near IFNL3, age, gender, insulin resistance, 25 (OH) vitamin D status, coinfection with HBV and/or HIV and liver fibrosis stage were associated with antiviral response (35). With new generation DAAs, SVR improved significantly in all those subgroups. The SVR is still lower in patients with advanced liver fibrosis or cirrhosis, while the number of these patients is increasing in coming decades (59). Hepatitis C Virus genotypes, cirrhosis, co-morbidities like hemodialysis, HIV/HCV coinfection and liver transplantation, history of previous treatment, treatment cost and contraindications (Table 1) are some of the important factors, which should be considered in selecting the appropriate treatment regimens. In addition to SVR, incidence of adverse events, drug resistance and drug-drug interaction can be considered for selecting the best regimen.
| Direct-acting Antiviral Agents | Contraindication/Warning |
|---|---|
| Daclatasvir | Drug Influencing CYP3A, Co-administration with Amiodarone |
| Sofosbuvir | Co-administration with Amiodarone, patients with estimated GFR of less than 30 mL/min/1.73 m2. Caution is needed for co-administration with beta blockers |
| Ledipasvir/Sofosbuvir | Co-administration with Amiodarone, inducers of p-glycoprotein, patients with estimated GFR of less than 30 mL/min/1.73 m2 |
| Velpatasvir/Sofosbuvir | Inducers of p-glycoprotein, patients with estimated GFR of less than 30 mL/min/1.73 m2, Co-administration with Amiodarone |
| Simeprevir | Cirrhotic patients with Child Pugh class B and C |
aSource: based on the 2015 AASLD and EASL guideline and product label information.
3.4.2. Management of Hepatitis C Virus Genotype 1
In the era of IFN therapy of HCV infection, HCV-G1 was the most intractable among all HCV genotypes, categorized as a difficult-to-treat genotype (60). Using IFN-free DAA regimens, HCV-G1 changed from the “difficult-to-treat” to the “easy-to-treat” genotype. The main barrier for using DAA was high cost, but today, high quality drugs with low cost are domestically manufactured in Iran.
The regimen of SOF/PegIFN/RBV for HCV-G1 was associated with an SVR of 73% in a real-world study (61), which was lower than the clinical trials. The data from clinical trials showed that SVR in treatment naive and non-cirrhotic patients with this regimen was more than 90% (62). Our meta-analysis showed that combination therapy with SOF plus PegIFN/RBV resulted in an SVR of 88.54% in patients with HCV-G1 infection (In press). However, detectable HCV RNA (more than 43 IU/mL) at week four was predicted to decrease the likelihood of achieving SVR for HCV-G1 patients over 60%. It is worth mentioning that around 95% of patients clear HCV RNA during four weeks of treatment with SOF/PegIFN/RBV regimen (37, 63, 64). Moreover, Kowdley et al. (63) showed that there is no significant difference between response to treatment with SOF/PegIFN/RBV regimen for 12 and 24 weeks (89% vs 89%). As a result, it seems that response-guided therapy plays no role in the treatment with SOF/PegIFN/RBV regimen.
In 2014, FDA approved the fixed-dose combination of LDV/SOF, as the first IFN-free all-oral HCV antiviral therapy with more than 95% efficacy, in non-cirrhotic patients with HCV-G1 infection (24, 25). Although the combination of LDV/SOF is more efficacious and more tolerable, this regimen is expensive in most countries. Fortunately, LDV/SOF is now available in Iran at a low cost. The LDV/SOF has been prescribed with or without RBV and for 12 or 24 weeks (24, 25), based on liver fibrosis, previous treatment experience, and RBV tolerability. Interestingly, in our meta-analysis (In Press), it was shown that treatment efficacy is comparable in all 12 or 24 weeks regimens with or without RBV, including 95% for 12 weeks of treatment with LDV/SOF, 96% for 24 weeks of treatment with LDV/SOF, 96% for 12 weeks of treatment with LDV/SOF/RBV and 98% for 24 weeks of treatment with LDV/SOF/RBV. In this study it was observed that cirrhosis (Child-Pugh A) could impact (OR = 0.21) the SVR12 only in the regimen of 12 weeks of treatment with LDV/SOF. As a result, it is recommended to treat patients with cirrhosis (Child-Pugh A) with LDV/SOF/RBV for 12 weeks or with LDV/SOF for 24 weeks based on the RBV contraindication and affordability of drugs while it seems treatment of non-cirrhotic patients with LDV/SOF for 12 weeks is reasonable. Based on WHO guideline, the regimen of DCV/SOF had better results than LDV/SOF, based on the rate of serious adverse events and treatment discontinuation, although the differences were not statistically significant. Other DAAs with high efficacy have also been introduced including PTV-r/OBV/DSV, SMV-containing regimens and GZR/EBR (35).
The current recommendation (51) for therapy in treatment-naive patients without cirrhosis, who are infected with HCV-G1, is 12 weeks of DCV/SOF or LDV/SOF. The only regimens that are currently approved for the treatment of HCV infection in patients with decompensated cirrhosis are LDV/SOF plus RBV (24 weeks) or DCV/SOF plus RBV (24 weeks), approved in Europe for patients with HCV-G1 and -G4 (65).
For treatment-naive and -experienced patients with HCV-G1 infection, LDV/SOF, PTV-r/OBV/DSV and DCV/SOF were superior to PegIFN-based treatments. The recommendations for treatment of HCV-G1 are summarized in Table 2.
| Non-Cirrhotic and Naive to SOF-Based Regimens | Non-Cirrhotic with History of SOF-Based Therapy | Compensated Cirrhosisa (Child A) and Naive to SOF-Based Regimens | Compensated Cirrhosisa (Child A) with History of SOF-Based Therapy | Decompensated Cirrhosis (Child B or C) |
|---|---|---|---|---|
| A. Daily DCV (60 mg) + Daily SOF (400 mg) for 12 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 12 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) for 24 weeks or plus Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) plus Daily weight adjusted RBV (1000 - 1200 mg) for 24 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks or without RBVb,c |
| B. Daily LDV (90 mg)+ Daily SOF (400mg) for 12 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) with Daily RBV (1000 - 1200 mg) for 12 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) for 24 weeks or plus Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) plus Daily weight adjusted RBV (1000 - 1200 mg) for 24 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) with Daily RBV (1000 - 1200 mg) for 24 weeks or without RBVb,c |
| C. As alternative: Daily SOF (400 mg) + Weekly PegIFN α-2a (180 µg) Or -2b (1.5 µg/Kg) + Daily weight adjusted RBV (1000-1200 mg) for 12 weeks |
Abbreviations: DCV, Daclatasvir; LDV, Ledipasvir; SOF, Sofosbuvir; RBV, Ribavirin.
aIncluding patients with pre-cirrhosis (F3-F4).
bThere is not any priority between suggested regimens above. Both regimens are available now.
c24 weeks without RBV in cases with RBV intolerance or contraindication.
3.4.3. Management of Hepatitis C Virus Genotype 2
The best first-line treatment option for patients infected with HCV-G2 is the combination of SOF plus RBV at divided doses for 12 weeks. Twelve weeks treatment with SOF and RBV led to an SVR12 of 80% among veterans with HCV-G2 infection, compensated cirrhosis, and multiple comorbidities, regardless of their treatment history (66). Other options may be useful in a small number of patients, who fail on this regimen (65). The recommendations for treatment of HCV-G2 are summarized in Table 3.
| Non-cirrhotic and Naive to SOF-Based Regimens | Non-Cirrhotic with History of SOF-Based Therapy | Compensated Cirrhosisa (Child A) and Naive to SOF-Based Regimens | Compensated Cirrhosisa (Child A) with History of SOF-Based Therapy | Decompensated Cirrhosis (Child B or C) |
|---|---|---|---|---|
| A. Daily SOF (400 mg) + Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 12 weeks | A. Daily SOF (400 mg) + Daily weight adjusted RBV (1000 - 1200 mg) for 24 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks or without RBVb |
| B. As alternative: Daily DCV (60 mg) + Daily SOF (400 mg) for 12 weeks | B. As alternative: Daily DCV (60 mg) + Daily SOF (400 mg) for 12 weeks | |||
| C. As alternative: Daily SOF (400 mg) + Weekly PegIFN α-2a (180 µg) Or -2b (1.5 µg/Kg) + Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeks |
Abbreviations: DCV, Daclatasvir; RBV, Ribavirin; SOF, Sofosbuvir.
aIncluding patients with pre-cirrhosis (F3-F4).
b24 weeks without RBV in cases with RBV intolerance or contraindication.
3.4.4. Management of Hepatitis C Virus Genotype 3
Patients infected with HCV-G3 are at a higher risk of HCC and faster progression to cirrhosis and despite progress in the development of well-tolerated, all-oral, interferon-free therapies, there are many un-answered and challenging questions regarding therapy of HCV-G3. The optimal regimen and duration of treatment in some groups remain unclear, especially for treatment-experienced patients.
Welzel and colleagues (67) investigated the safety and efficacy of SOF/RBV in patients with HCV-G3, including those who had previously received IFN-based therapy or had cirrhosis. Overall SVR12 was 62%. The SVR was the lowest in treatment-experienced patients with cirrhosis (43%). Sofosbuvir plus PegIFN/RBV in treatment-naive patients increased SVR to 92%, but it was associated with adverse events (68). However, SOF-containing regimen with PegIFN/RBV has the benefit of shortening duration of therapy to 12 weeks. Furthermore, SOF/PegIFN/RBV can be recommended in non-cirrhotic patients with HCV-G3 as the second option if DCV/SOF is not available (69). The SVR for LDV/SOF and LDV/SOF/RBV in HCV-G3 is 62.5% and 81%, respectively. Sofosbuvir/LDV is not an ideal treatment for HCV-G3.
Preliminary data of DCV/SOF combination, showed SVR12 of 70% (with RBV) and 71% (without RBV) in decompensated cirrhosis (70). However, the data of the ALLY-3 trial demonstrated a SVR12 of 90% and 86% for treatment-naive and treatment-experienced patients, respectively (48). This regimen has been approved for HCV-G3 in the United States. However, the low SVR in cirrhotic patients mandates prolongation of therapy to 24 weeks and addition of RBV. The DCV/SOF combination is the best oral therapy option and the addition of RBV does not appear to be needed in non-cirrhotic patients (49, 71). The recommendations for treatment of HCV-G3 are summarized in Table 4.
| Non-cirrhotic and Naive to SOF-Based Regimens | Non-Cirrhotic with History of SOF-Based Therapy | Compensated Cirrhosisa (Child A) and Naive to SOF-Based Regimens | Compensated Cirrhosisa (Child A) with History of SOF-Based Therapy | Decompensated Cirrhosis (Child B or C) |
|---|---|---|---|---|
| A. Daily DCV (60 mg) + Daily SOF (400 mg) for 12 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 12 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks or without RBVb |
| B. As alternative: Daily SOF (400 mg) + Weekly PegIFN α-2a (180 µg) Or -2b (1.5 µg/Kg) + Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeks | ||||
| C. As alternative: Daily SOF (400 mg) + Daily weight adjusted RBV (1000 - 1200 mg) for 24 weeks |
Abbreviation: DCV, Daclatasvir; RBV, Ribavirin; SOF, Sofosbuvir.
aIncluding patients with pre-cirrhosis (F3-F4).
b24 weeks without RBV in cases with RBV intolerance or contraindication.
3.4.5. Management of Hepatitis C Virus Genotype 4
The all-oral regimen of LDV/SOF and DCV/SOF are effective and safe for HCV-G4 in both treatment-naive and -experienced patients, including those with compensated cirrhosis (72). We have limited data regarding combination of DCV/SOF in patients with HCV-G4. Nevertheless, given the antiviral effectiveness of both DCV/SOF against this genotype in vitro, it is likely that the results in patients infected with HCV-G1 can be extrapolated (65). In one study, HCV-G4 infected patients were treated by DCV/SOF with or without RBV for 12 or 24 weeks. The SVR12 was achieved in different treatment regimens as follows: DCV/SOF for 12 weeks (84.12%), DCV/SOF for 24 weeks (87.5%), DCV/SOF/RBV for 12 weeks (92.72%) and finally SOF/DCV/RBV for 24 weeks (96.77%) (73). The recommendations for treatment of HCV-G4 are summarized in Table 5.
| Non-cirrhotic and Naive to SOF-Based Regimens | Non-Cirrhotic with History of SOF-Based Therapy | Compensated Cirrhosisa (Child A) and Naive to SOF-Based Regimens | Compensated Cirrhosisa (Child A) with History of SOF-Based Therapy | Decompensated Cirrhosis (Child B or C) |
|---|---|---|---|---|
| A. Daily DCV (60 mg) + Daily SOF (400 mg) for 12 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 12 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) for 24 weeks or plus Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) plus Daily weight adjusted RBV (1000 - 1200 mg) for 24 weeksb | A. Daily DCV (60 mg) + Daily SOF (400 mg) with Daily RBV (1000 - 1200 mg) for 24 weeks or without RBVb,c |
| B. Daily LDV (90 mg)+ Daily SOF (400mg) for 12 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) with Daily RBV (1000 - 1200 mg) for 12 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) for 24 weeks or plus Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) plus Daily weight adjusted RBV (1000 - 1200 mg) for 24 weeksb | B. Daily LDV (90 mg)+ Daily SOF (400mg) with Daily RBV (1000 - 1200 mg) for 24 weeks or without RBVb,c |
| C. As alternative: Daily SOF (400 mg) + Weekly PegIFN α-2a (180 µg) Or -2b (1.5 µg/Kg) + Daily weight adjusted RBV (1000 - 1200 mg) for 12 weeks |
Abbreviations: DCV, Daclatasvir; LDV, Ledipasvir; RBV, Ribavirin; SOF, Sofosbuvir.
aIncluding patients with pre-cirrhosis (F3-F4).
bThere is not any priority between suggested regimens above. Both regimens are available now.
c24 weeks without RBV in cases with RBV intolerance or contraindication.
3.4.6. Management of Hepatitis C Virus in Patients with Human Immunodeficiency Virus/Hepatitis C Virus Coinfection
It has been estimated that about 2.3 million people are infected with both HIV and HCV (74). These coinfected patients have more rapid progression towards advanced liver fibrosis and decompensation compared with those with HCV-monoinfection. Therefore, all patients with HIV/HCV coinfection should be considered for HCV treatment (75, 76) and SVR is the same for co-infected and mono-infected individuals. Previously, treatment of HIV/HCV patients with PegIFN has led to major side effects like depression, weight loss, severe anemia, thrombocytopenia and neutropenia. In addition, SVR among these patients was lower than HCV-mono-infected patients (77). Currently, treatment with DAAs has substantially simplified treatment of HIV/HCV coinfection with actual SVR rate and minimal side effects (78). The most important issue regarding HCV/HIV treatment with DAAs is related to drug-drug interaction with antiretroviral therapy. Table 6 shows the most important drug-drug interaction.
| Direct-acting Antiviral Agents | Interaction |
|---|---|
| Daclatasvir | Potential interaction with Efavirenz, Nevirapine and Ritonavir |
| Ledipasvir/Sofosbuvir | Potential interaction with Tenofovir and Efavirenz |
| Simeprevir | It should not be used with Efavirenz, Nevirapine, Lopinavir and Ritonavir |
| Grazoprevir/Elbasvir | It should not be used with cobicistat, efavirenz, etravirine, nevirapine, or any HIV protease inhibitor |
| Velpatasvir/Sofosbuvir | Potential Interaction with Tenofovir-DF. It should not be used with Efavirenz and Nevirapine |
| Sofosbuvir | None |
aDrug-Drug interaction were reported here are beween DAAs and these drugs: Abacavir, Emtricitabine, Lamivudine, Tenofovir, Zidovudine, Dolutegravir, Efavirenz, Nevirapine, Lopinavir and Ritonavir.
bSource: University of Liverpool, hepatitis drug interactions webpage (http://www.hep-druginteractions.org).
3.4.7. Management of Hepatitis C Virus in Patients with Chronic Kidney Disease
Hepatitis C Virus infection is prevalent among hemodialysis patients and they are at risk of transmitting HCV. Therefore, they should be prioritized for HCV treatment (65). Briefly, HCV treatment among patients with chronic kidney disease should be performed based on Creatinine Clearance (CRCL). Sofosbuvir, which is used in many DAA regimens cannot be administered in patients with severe renal failure (CRCL < 30 mL/min/1.73 m2) (77).
Based on the 2015 AASLD guideline (51), for patients with CRCL between 30 and 80 mL/minute, no dose adjustment is needed. This means that DCV (60 mg), SMV (150 mg), SOF (400 mg), fixed dose combination of LDV (90 mg)/SOF (400 mg) and fixed dose combination of VEL (100mg)/ SOF (400mg) can be applied in these patients.
For patients with CRCL of less than 30 mL/min/1.73 m2 and infected with HCV-G1 and -G4, the regimen of EBR (50 mg) plus GRZ (100 mg) for 12 weeks can be used. For other genotypes (2, 3, 5 and 6) PegIFN plus dose adjusted RBV at 200 mg daily can be used. The RBV should be discontinued if hemoglobin level declines more than 2 g/dL despite administration of erythropoietin.
3.4.8. Management of Hepatitis C Virus in Patients with Liver Transplantation
Recurrence of HCV infection among patients, who undergo liver transplantation with detectable HCV RNA is still a major concern with morbidity and allograft loss after transplantation. Approximately 30% of HCV transplanted patients develop acute severe recurrent hepatitis progressing rapidly to liver cirrhosis and increased risk of death (79). In patients with end stage liver disease in the waiting list for liver transplantation, it is advised to start DAA as soon as possible (80). It has been shown that the chance of HCV recurrence post-transplantation would be reduced if HCV RNA is undetectable at least 30 days before transplantation (81). In the case of post liver transplantation recurrence of HCV, treatment should be started two to four months after liver transplantation to pass the initial critical period. For years, IFN-based therapy including PegIFN with or without RBV had been widely used against recurrent HCV after liver transplantation. Unfortunately, these regimens were poorly tolerated and the SVR in these patients was reported to be only 20% - 50%, with significant adverse effects (82). After the advent of DAAs, satisfactory virological responses with minimal side effects were observed in this setting. Various regimens, including 12 to 24 weeks treatment with SOF-based regimens including DCV/SOF, LDV/SOF and SMV/SOF with or without RBV have satisfactory responses in more than 80% of the post-transplant patients (83-85). The treatment regimens for post liver transplant patients with different HCV genotypes are showed in Table 7.
| Patients Group | HCV Genotype 1 or 4, Treatment Naive or Experienced | HCV Genotype 2, Treatment Naive or Experienced | HCV Genotype 3, Treatment Naive or Experienced |
|---|---|---|---|
| Non-cirrhotic or compensated cirrhosis | A. Daily fixed-dose combination of LDV (90 mg)/SOF (400 mg) with weight-based RBV for 12 weeks | A. Daily DCV (60 mg)/SOF (400 mg), with low initial dose of ribavirin for 12 weeks | A. Daily DCV (60 mg)/SOF (400 mg) with low initial dose of ribavirin for 12 weeks |
| B. Daily DCV (60 mg)/SOF (400 mg) with low initial dose of RBV for 12 weeks | B. Daily SOF (400 mg) and weight-based RBV for 24 weeks | ||
| Decompensated cirrhosis | A. Daily fixed-dose combination of LDV (90 mg)/SOF (400 mg) with low initial dose of ribavirin for 12 weeks | A. Daily SOF (400 mg) and RBV (initial dose 600 mg/day, increased as tolerated to weight-based dose) for 24 weeks. | - |
| RBV ineligible | A. Daily fixed-dose combination of LDV (90 mg)/SOF (400 mg) for 24 weeks | A. Daily DCV (60 mg)/ SOF (400 mg) for 24 weeks | A. Daily DCV (60 mg)/ SOF (400 mg) for 24 weeks |
| B. Daily DCV (60 mg)/ SOF (400 mg) for 24 weeks |
Abbreviations: DCV, Daclatasvir; LDV, Ledipasvir; RBV, Ribavirin; SOF, sofosbuvir.
Despite excellent response to treatment of HCV after liver transplantation, many groups have suggested treatment of hepatitis in patients, who are candidate for transplantation and are on the waiting list awaiting transplantation. The regimen best works in patients with compensated cirrhosis, however, some patients with decompensated disease have shown excellent response with clearance of the virus and decrease in their disease severity (Model for End-stage Liver Disease, MELD score), not needing a transplant. Here, it should be cautioned that patients, who improve under the therapy but not enough to be delisted, may be disadvantaged because of moving down on the waiting list and further delays in their transplantation. A variety of regimens established for treatment of HCV infection after liver transplantation work in the pre-transplant setting as well (Table 7).
4. Conclusions
Low cost DAAs and increasing HCV diagnosis and treatment rate are crucial for HCV elimination programs. We believe that health insurances can help expand treatment programs. Hepatitis C Virus screening system should be designed, with more focus on high-risk populations.
In the near future and with availability of low cost DAA, HCV elimination seems to be possible in Iran if infrastructure for enhanced diagnosis and treatment uptake is provided. Increasing insurance coverage and consequently lower patient co-payment for DAA will help expand access to therapy among HCV-infected patients. High coverage screening programs, with a main focus on groups at a higher risk of HCV infection, like IDUs, are required. These screening programs should be designed based on the current human resources in the public health system such as Behvarz and general practitioners (GPs). In addition, GPs can be trained for HCV therapy in targeted seminars and then they can be guided via telecommunication for more HCV therapy all over the country (86).
Increasing diagnosis and treatment uptake can make HCV elimination, a real possibility in Iran. While increasing efficacy has moderate declines in all HCV-related indicators, an aggressive treatment strategy would eliminate HCV in Iran, bringing the viremic prevalence to approximately 0.02% by 2030 (8).
Currently, access to DAAs is limited in the world, with treatment rates being lowest in most resource-limited countries, including those countries with the highest prevalence. Fortunately, we have access to DAAs in Iran. The use of oral DAAs has the potential to provide treatment scale-up due to simplified drug regimens, laboratory assessments during treatment, and service delivery models. Key desirable characteristics of future HCV treatment regimens include high efficacy, high tolerability, pan-genotype activity, short treatment duration, oral therapy, affordability, and availability as fixed-dose combination. Using such a regimen, HCV treatment delivery could be greatly simplified. Treatment could be initiated following confirmation viremia, with an initial assessment of the stage of liver disease. A combination of DAA therapy that is safe and effective across genotypes could remove the need for intermediate HCV RNA level assessments for response-guided therapy and reduce the need for adverse event monitoring. Simple, safe and short therapy will also facilitate simplified service delivery, including task shifting, decentralization, and integration of treatment and care. The opportunity for HCV treatment scale up using such delivery approaches will depend on efforts needed to guarantee that the new DAAs are affordable in low-income settings. This will require the engagement of all stakeholders, including, clinicians, and companies developing these new treatments, Ministry of Health, health insurance companies, and community-based organizations.