1. Introduction
Hepatitis C virus (HCV) is an enveloped single stranded RNA positive sense virus which is classified in the genus Hepacivirus of the Flaviviridae family. It is a major cause of chronic liver disease, possibly leading to liver cirrhosis and hepatocellular carcinoma (1, 2). In the majority of the HCV infected patients, the infection becomes chronic, but in 14% - 45% of the cases, the virus is spontaneously cleared (3). The last estimates of the disease burden show an increase in seroprevalence over the last 15 years to 2.8%, equating to more than 185 million infections worldwide (4). However, the prevalence rate of HCV in Iran is low, where the rate in the general population is < 0.5% (5, 6).
The virus genome encodes a polyprotein of approximately 3000 amino acids that is flanked by 5′ and 3′ non-coding regions (5′-NCR and 3'-NCR) (7). Translation of the polyprotein is mediated by an internal ribosome entry site (IRES) embedded within the 5′-NCRs, and the individual viral proteins are produced upon cleavage of the polyprotein by host and viral proteases. These include three structural proteins (core, E1, and E2), the p7 protein, and six non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B), which are involved in virion assembly and viral RNA replication (8). The virus is classified into seven different genotypes and more than a hundred subtypes (termed a, b, c, etc.), and it also manifests itself in the sera or tissues of patients with the appearance of quasispecies. The common treatment method for HCV infection is pegylated interferon a-2a and ribavirin (peg-IFNa-2a/RBV) combination therapy in developing countries (1).
This treatment is used to eradicate the infection and reduce the risk of cirrhosis and hepatocellular carcinoma (9). Treatment results have been shown to be affected by various viral factors such as the HCV genotype or pretreatment viral load and host factors such as fibrosis, cirrhosis, liver steatosis, and insulin resistance (10, 11). Recently, there has been evidence showing that a genetic polymorphism near the interleukin-28B gene encoding IFN-λ3 is correlated with the treatment response (12, 13).
HCV infection is related to various immunological abnormalities, including the production of organ specific and non-organ specific autoantibodies (autoAbs) (14). Organ specific autoAbs consist of those produced against their targets in pancreatic islet cells (15), thyroid (16), the adrenal cortex (15), and gastric parietal cells (17). Non-organ specific autoAbs cover anti-smooth muscle Abs, anti-nuclear Abs (ANA), anti-neutrophil cytoplasmic Abs, anti-mitochondrial Abs, and anti-liver-kidney microsomal Abs (18).
These autoAbs are mainly divided into five groups: i) autoAbs as serological markers for autoimmune liver disease; ii) autoAbs as serological markers for extra-hepatic autoimmune diseases; iii) autoAbs to endocrine organs; iv) autoAbs to tumor-associated antigens, and v) other autoAbs (19).
Since HCV penetrates into the hepatocytes through the binding of its envelope protein (E2) to CD81, which is also expressed in B-lymphocytes, Pileri et al. proposed that HCV binding to B-cells lowers their activation threshold and because of that, the production of autoAbs is facilitated (20). On the other hand, regarding peg-IFNa-2a/RBV combination therapy, the cornerstones of the control of HCV infection have immunomodulatory effects (21), such as the production of autoantibodies (22). A novel autoAb that recognizes special cytoplasmic rod and ring (RR) structures was first identified in the sera of HCV patients that were studied using commercial ANA slides (23). Inosine monophosphate dehydrogenase 2 (IMPDH2) and cytidine triphosphate synthase 1 (CTPS1) were identified as potential autoAbs targets, which are localized to the RR structures (23). Anti-rod and ring (anti-RR) autoAbs were specific to HCV patients who were under peg-IFNa-2a/RBV combination therapy (24).
2. Objectives
The aim of the present study was to determine the relationship between the presence of autoantibodies to the cytoplasmic rod and ring particles in the serum of patients with chronic hepatitis C and the response to peg-IFNa-2a/RBV combination therapy.
3. Methods
3.1. Study Population
The study was conducted on samples gathered from 120 chronic hepatitis C patients who referred to the digestive disease research institute (DDRI) of Shariati hospital affiliated to Tehran University of Medical Sciences (TUMS) from 2010 to 2012. The samples were stored at -80°C. The exclusion criteria included coinfection with human immunodeficiency virus (HIV) and hepatitis B virus (HBV) (25). The current study was approved by the Ethics Committee of Iran University of Medical Sciences (IUMS), Tehran, Iran.
3.2. Treatment Method
The patients were treated with standard combination therapy using pegylated IFNa-2a (Peg-IFNa-2a; Pegasys, Roche, Basel, Switzerland), 180 µg per week, in combination with ribavirin (RBV) (Copegus, Roche), 1,000 - 1,200 mg per day. HCV infected patients with genotypes 1 and 4 were treated for 48 weeks, whereas genotype 3-HCV positive patients were treated for 24 weeks according to standard methods for HCV therapy (26).
3.3. Explanation of Response
To specify the types of response to peg-IFNa-2a/RBV combination therapy, the presence of genomic HCV RNA was evaluated at the end of the therapy and 6 months thereafter. The hepatitis C viral load was measured in the plasma samples. According to the results of the HCV-RNA evaluation at the aforesaid time points, the cases were classified into three groups: sustained virological response (SVR) that was named because of undetectable HCV RNA at the end of 24 weeks; End-of-treatment (EOT) response that was tested at 24 weeks after initiation of treatment for HCV genotype 2 and 3 infections and at 48 weeks for genotype 1 of HCV; and Relapse response that happened when HCV RNA reemerged in a patient who previously had obtained an EOT response. All patients with detectable hepatitis virus RNA, at the end of the treatment and during follow-up, were considered as non-responders (NR) (26, 27).
3.4. Indirect Immunofluorescence Assay (IIFA)
All samples were analyzed by IIF assay with HEp-2 slides from Euroimmun (Lu¨ beck, Germany) at a screening dilution of 1/80 according to standard IIF protocol (28) with EUROStar III PlusFlurescent microscope under 400 magnifications. Analyses were done separately by two expert observers of Firouzgar Hospital affiliated to Iran University of Medical Sciences (IUMS). Images were captured using a BASLER acA2500-14uc-Imager.
3.5. RNA Extraction and Genotyping
To determine HCV genotypes, viral RNA was extracted from 140 μL of sera using the QIAamp Viral RNA Extraction kit (Qiagen GmbH, Hilden, Germany) based on the manufacturer’s protocol. HCV-RNA in sera was detected by the reverse transcription-nested polymerase chain reaction (RT-nested PCR) assay (29). The HCV genotypes were analyzed in HCV-positive samples using restriction fragment length polymorphism (RFLP) assay of the 5′-NCR of the HCV genome as described previously (30).
3.6. Statistical Analysis
Statistical analyses were performed using SPSS version 17 (SPSS, Chicago, IL). Descriptive indices, Fisher’s exact test, and Chi-square test were used. P values < 0.05 were considered statistically significant.
4. Results
120 patients chronically infected with HCV were enrolled in the current study and divided into three groups. The groups were defined as SVR (n = 40), relapsers (n = 40), and spontaneous clearance (n = 40). The mean age (±SD) of the patients was 43 ± 13.2 (range of 17 - 71 years). Among 120 patients, 90 (75%) were male. Clinical data on biochemical parameters, virological aspects, and type of therapeutic response (using One-way ANOVA and Chi-square test) are presented in Table 1.
| Patients | SVRb | Relapse | SCc | Total | P Value |
|---|---|---|---|---|---|
| No. of patients | 40 (33 ) | 40 (33 ) | 40 (33 ) | 120 (100) | |
| Gender Male/female | 34/6 | 32/8 | 24/16 | 90/30 | 0.024 Chi-square test |
| Age, y | 37.6 ± 10.7 (17 - 67) | 43.8 ± 13.04 (19 - 69) | 59.2 ± 6.4 (53 - 71) | 43 ± 13.1 (17 - 71) | < 0.001One-way ANOVA |
| Laboratory Parameters | |||||
| Pre ALTd, IU/L | 78.2 ± 59.2 (18 - 275) | 72.8 ± 41.1 (17 - 197) | 15.2 ± 9.1 (1 - 41) | 61 ± 51.4 (1 - 275) | < 0.001a One-way ANOVA |
| Post ALT, IU/L | 26.1 ± 13 (9 - 60) | 34.9 ± 20.4 (8 - 94) | 15.4 ± 8.5 (2 - 40) | 30.4 ± 17.5 (8 - 94) | < 0.001 One-way ANOVA |
| Pre AST, IU/L | 53.6 ± 47.2 (19 - 309) | 60.4 ± 35.8 (15 - 172) | 16.4 ± 6.2 (3 - 29) | 47.2 ± 40.5 (3 - 309) | < 0.001 One-way ANOVA |
| Post ASTe, IU/L | 27.6 ± 12.1 (14 - 67) | 35.3 ± 19.9 (8 - 106) | 16.5 ± 6.2 (4 - 29) | 31.4 ± 16.7 (8 - 106) | < 0.001 One-way ANOVA |
| Pre ALPf, IU/L | 197.3 ± 67.5 (76 - 364) | 231.1 ± 77.8 (87 - 415) | 230.1 ± 54.7 (153 - 349) | 217.9 ± 70.1 (76 - 415) | 0.061 One-way ANOVA |
| Post ALP, IU/L | 189 ± 56.2 (90 - 329) | 207.7 ± 81.5 (62-414) | 230.3 ± 54.6 (156 - 350) | 198.3 ± 70 (62 - 414) | 0.056 One-way ANOVA |
| Pre GGTg, IU/L | 40.5 ±23.2 (14 - 122) | 76.1 ± 61.7 (12-247) | 23.6 ± 13 (11-67) | 49.2 ± 45.4 (11 - 247) | < 0.001 One-way ANOVA |
| VLDLh, mmol/L | 24.7 ± 23.4 (7 - 130) | 22 ± 10.1 (4 - 40) | 20 ± 13.2 (6-89) | 23.3 ± 17.6 (4 - 130) | 0.578 One-way ANOVA |
| Clinical Information | |||||
| No. of Cirrhotic patients | 0 | 3 (7.5) | 0 | 3 (2.5) | 0.046 Chi-square test |
| HCV Genotypes/Subtypes | |||||
| 1a | 19 (47.5) | 17 (42.5) | 0 | 36 (30) | < 0.001 Chi-square test |
| 1a/1b | 0 | 0 | 4 (10) | 4 (3.3) | |
| 1b | 2 (5) | 5 (12.5) | 0 | 7 (5.8) | |
| 2b | 1 (2.5) | 0 | 0 | 1 (0.8) | |
| 3a | 18 (45) | 13 (32.5) | 0 | 31 (25.8) | |
Clinical Information of the Patients and Various Types of Therapeutic Responses to Pegylated- Interferon Alfa-2a and Ribavirin Combination Therapya
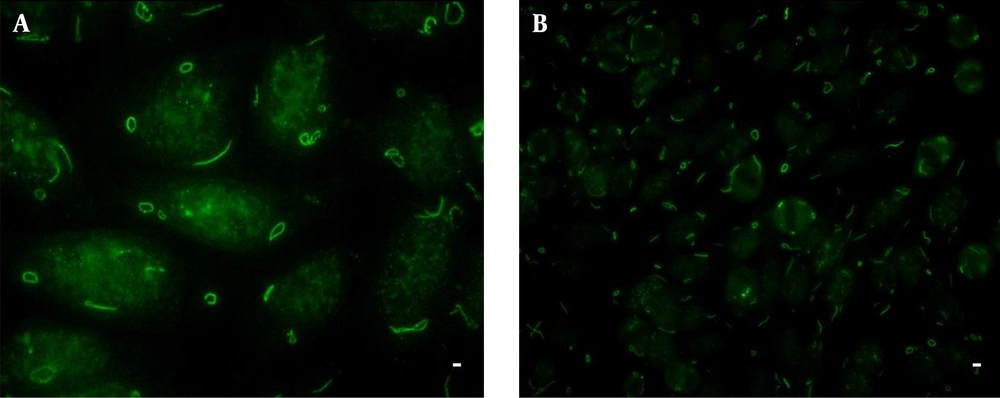
Anti-rod and ring antibodies (Anti-RR Abs) were readily identified on Euroimmun HEp-2 slides. The abovementioned autoantibodies were detected in only one serum of the 120 patients under the study (0.8%) that belonged to one out of 40 patients with relapse response (2.5%) (Figure 1). No correlation was found between the type of response to peg-IFNa-2a/RBV combination therapy and the presence of anti-RR Abs (P = 0.365; Chi-square test). However, other IIF-HEp-2 patterns were observed in variable proportions in all groups of patients. The speckled cytoplasmic pattern was the dominant pattern in the studied patients (Table 2).
| Types of Responses | ||||
|---|---|---|---|---|
| IIF-HEp-2 Patternsb | SVRc (n = 40) | Relapse (N = 40) | SCd (N = 40) | P Value |
| Nucleoli | 4 (10) | 11 (27.5) | 6 (15) | 0.105 Chi-square test |
| FSe | 4 (10) | 2 (5) | 2 (5) | 0.585 Chi-square test |
| Midbody | 0 | 3 (7.5) | 2 (5) | 0.232 Chi-square test |
| SCPf | 17 (42.5) | 15 (37.5) | 11 (27.5) | 0.362 Chi-square test |
| Mitotic spindle | 0 | 1 (2.5) | 3 (7.5) | 0.164 Chi-square test |
| Rods and rings | 0 | 1 (2.5) | 0 | 0.365 Chi-square test |
| Overall IIF-Hep-2 Reactivity | 25 (62.5) | 33 (82.5) | 24 (60) | |
Distribution of Patients with Hepatitis C Virus and Other Hepatic and Non-Hepatic Diseases According to the Presence of Anti-Rod and Ring Antibodies and Other Indirect Immunofluorescencee-HEp-2 Patternsa
Background information regarding autoimmune disorders in the patients was also studied. Two patients (1.7%) were positive, 77 patients (64.2%) were negative, and the data of 41 patients (34.2%) were unknown. Among positive patients, none of them had anti-RR reactivity; thus, any association was not observed between having an autoimmune disease and the presence of anti-RR Abs (P = 1.000; Fisher’s exact test).
The data of the present study show that only one patient had anti-RR reactivity, and 119 patients presented other IIF-HEp-2 patterns or were negative for the IIF-HEp-2 test.
4. Discussion
This study showed that HCV infection is associated with the type of immune reactions, part of which is reflected by the presence of organ and non-organ specific autoAbs. Probable mechanisms for the production of auto Abs may include molecular mimicry, an interaction between the HCV and B cells which boosts their reproduction, or direct infection of the immune cells by HCV (31).
In one study by Keppeke et al. cell cultures treated with ribavirin demonstrated that this drug has the potential to induce the formation of the RR pattern; in other words, only HCV positive patients, treated with peg-IFNa-2a/RBV combination therapy, had anti-RR Abs, whereas none of the untreated ones showed this pattern (28). In this study, none of the samples from the spontaneous clearance patients without any treatment method were positive for an RR pattern (28).
In another study by Felisberto et al. the presence of anti-RR Abs was observed just after the beginning of treatment. In 6% of patients, its appearance started within the first month; however, after 6 months, > 47% of the samples presented with the RR pattern. Another finding of the study was that, among anti-RR-positive patients, 77% did not respond to the treatment. On the other hand, among anti-RR-negative patients, the rate was 64%. Thus, this pattern is not related to the success of peg-IFNa-2a/RBV combination therapy (32).
The presence of autoantibodies to cytoplasmic RR particles in the serum of patients with chronic HCV Infection has been reported in various populations around the world. For example, it was detected in 14.1% of 342 HCV infected patients and 38% of 108 patients receiving Peg-IFN/RBV, but it was present in none of 166 untreated patients (28). In the study by Covini et al. analyses revealed the presence of antibodies to rod-like cytoplasmic structures in 15 out of 75 (20%) patients with chronic hepatitis C. Anti-RR antibodies were found in sera collected only during Peg-IFN/RBV treatment; but they were never detected before antiviral therapy or even in control groups. However, the antibodies were detected more often in non-responder/relapsers than responder patients (33% versus 11%) (24). In another study, about 20-35% of HCV patients treated with Peg-IFN/RBV combination therapy were known to induce the production of anti-RR antibodies after several weeks or months (24, 33).
In the current study, the RR pattern was only observed in one case out of 120 HCV patients (0.8%) that was classified in the relapse group. The RR pattern was not shown in any of subjects who have been categorized in the SVR and spontaneous clearance groups. No correlation was found between the success of peg-IFNa-2a/RBV combination therapy and the presence of anti-RR Abs. The low prevalence of anti-RR in the Iranian HCV patients is intriguing and maybe related to genetics and environmental factors; however, there is no follow-up information in this regard here. Therefore, it seems that more studies need to be done in this area.
In conclusion, the present study independently confirmed the association of the IIF-HEp-2 RR pattern with HCV and found no association between the presence of anti-RR Abs and different groups in responding to the peg-IFNa-2a/RBV combination therapy. In addition, the current study was able to limit the trigger of anti-RR reactivity to the peg-IFNa-2a/RBV combination therapy. On the other hand, no association was detected between demographic parameters, response to treatment, HCV genotype, and having an autoimmune disease. Despite the clear association of anti-RR reactivity and peg-IFNa-2a/RBV combination therapy, it is striking that the majority (98.8%) of Iranian patients under the mentioned therapy showed no anti-RR reactivity.
According to this study, the rate of the presence of anti-RR Abs is extremely rare in Iranian patients treated with Peg-IFN/RBV. On the other hand, the genetic background could be an important factor for this discrepancy, which needs more comprehensive studies and research.