1. Background
Cardiovascular diseases (CVDs) are the leading cause of death and disability, both in developed and developing countries (1). Forty-two percent of all deaths in North Africa and the Middle East were reported to be attributable to CVDs (2). Based on a previous study, CVDs accounted for nearly 40% of mortality in Tehran, the capital city of Iran (3). A study of a random sample of 3723 subjects from an Iranian population demonstrated that 11.3% of the population had coronary symptoms and about 1.4% had experienced myocardial infarctions (3, 4).
CVD-related risk factors have been carefully examined in recent decades, and several demographic, anthropometric, and laboratory risk factors have been identified. Some studies provided evidence that elevated levels of liver enzymes, particularly gamma glutamyl transferase (GGT), could be used to predict type 2 diabetes mellitus, ischemic heart disease, and stroke incidence (5-8). Future cardiovascular events can be predicted using risk-assessment tools. The Framingham risk score (FRS) is a well-known tool, which is used worldwide in the prediction of 10-year cardiovascular risks (9). The American College of Cardiology and American Heart Association (ACC/AHA) jointly developed a newer tool recently to predict 10-year cardiovascular events (10). The FRS has been shown to have relatively high sensitivity and high negative predictive value in the prediction of CVD in nonalcoholic fatty liver disease (NAFLD) (11). Liver enzymes, particularly GGT, have been shown to have predictive capability in CVD.
2. Objectives
The aim of the present study was to determine the relationship between liver enzymes and the 10-year CVD risks predicted by FRS and ACC/AHA tools.
3. Methods
3.1. Study Population
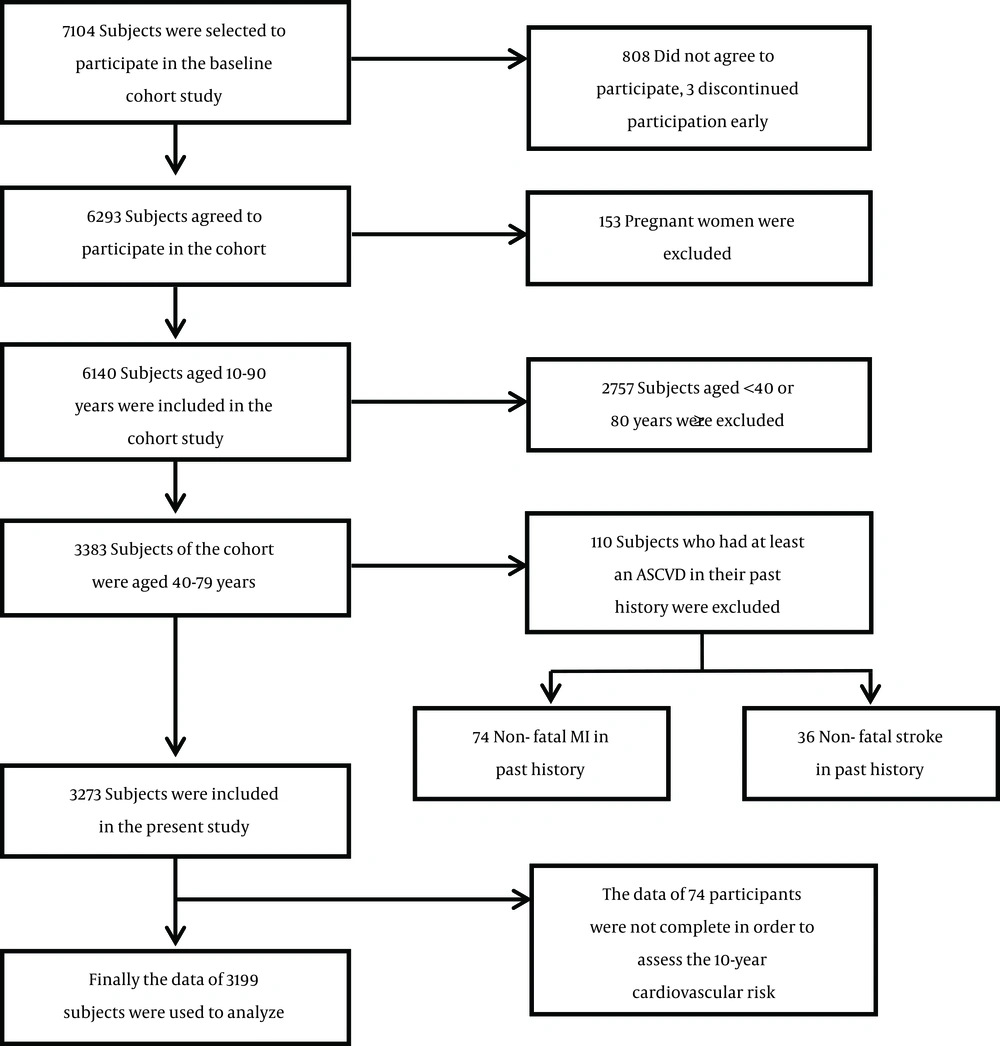
Baseline data from a population-based cohort study of Amol, a city in northern Iran, were used. In total, 6140 subjects between the ages of 10 - 90 years participated in the cohort study. Details on the sampling have been explained elsewhere (12). Informed consent was obtained from all the participants and the study was approved by the ethics committee of Iran University of Medical Sciences (registration number: IR.IUMS.REC.1392.19753). The final study consisted of data on 3199 subjects aged 40 - 79 years. A schematic diagram of the study population is presented in Figure 1.
3.2. Data Collection
The participants’ weights, heights, and blood pressure values were measured in health centers. To obtain more accurate data, the participants removed excess clothes and shoes during these measurements. The height measurements were made with the participants standing in an upright position and with their heels and buttocks against a wall. Blood pressure was measured using a fitted cuff, with the participants in a sitting position. Before measuring their blood pressure, the participants were asked to rest for at least 5 minutes in a quiet room. Systolic and diastolic blood pressures were determined as the first appearance and disappearance of Korotkoff sounds, respectively.
A venous blood sample was drawn from each participant following a 12-hour fast to assess fasting blood sugar and lipid profiles. All the tested parameters, including fasting blood sugar, triglycerides, high-density lipoprotein, low-density lipoprotein (LDL), and cholesterol were assessed enzymatically using a BS200 auto analyzer (Mindray, China). An automatic spectrophotometer was used to measure the enzymes. Serum insulin levels were measured by a chemiluminescence enzyme immunoassay method and homeostasis model assessment insulin resistance (HOMA-IR) values were calculated using the following formula: HOMA-IR = (fasting insulin [mL U/L] × fasting glucose [mg/dL])/405. Ten percent of the blood samples were evaluated by the Iranian national reference laboratory, with coefficients of variation between 1.7 and 3.8% reported for all laboratory tests.
Ten-year CVD risks were calculated separately for men and women using ACC/AHA equations and the Framingham risk-assessment tool for use in primary care (10, 13). In the assessment using the ACC/AHA tool, the gender-specific non-Hispanic American white version of the pooled cohort multivariate equations were used to calculate the 10-year risk of a first atherosclerotic CVD event, including coronary heart disease, death, nonfatal myocardial infarctions, and fatal or nonfatal strokes.
Using the Framingham tool, the 10-year risks were calculated separately for men and women according to the method used by D’Agostino et al., with age, total cholesterol, high-density lipoprotein, systolic blood pressure (treated or untreated), smoking (current smoker = 1, nonsmoker = 0), and diabetes (diabetes = 1, non-diabetes = 0) variables included in the model (13).
3.3. Statistical Analysis
A logistic regression analysis was conducted to determine the association between liver enzymes -including alkaline phosphatase (ALP), alanine aminotransferase (ALT), and GGT as well as 10-year CVD risks. Simple Logistic regression analyses were conducted separately for men and women, using the 10-year CVD risk (≥ 7.5%, ≥ 10%, and ≥ 20% with the ACC/AHA tool and ≥ 10% and ≥ 20% with the Framingham tool) as an outcome variable and liver enzymes, NAFLD, LDL, TG, DBP, HOMA-IR, and body mass index (BMI) as potential predictors of CVD. In the multiple logistic regression analyses, in addition to liver enzymes, NAFLD, LDL, TG, DBP, HOMA-IR, and body mass index (BMI) were entered as confounding variables. It is worth noting that Framingham and ACC/AHA tools do not directly use these variables to estimate 10-year CVD risks. The multiple logistic regression analyses were performed separately for men and women in which 10-year CVD risks (≥ 7.5%, ≥ 10%, and ≥ 20%) were considered outcomes. Prior to conducting the multiple logistic regression analyses, the multicollinearity of all potential predictors was checked using a multiple regression analysis. In the multicollinearity test, aspartate aminotransferase (AST) showed a low tolerance and thus was removed. Multicollinearity was checked again using remaining predictors. The tolerance value of all the independent variables was acceptable (> 0.2). As a result, there was little overlap or shared variance between the independent variables in model. Consequently, except for AST, all the predictors were included in the multivariate logistic model.
Odds ratios and significance levels were calculated for all the potential predictors. All the analyses were performed using SPSS 21 (IBM Corporation, Somers, NY, USA).
4. Results
Basic characteristics of the study population are shown in Table 1. In the study, 56.1% of the participants were men.
| Characteristics | Men | Women | P Value |
|---|---|---|---|
| Agea (years) | 53.94 ± 9.37 | 53.51 ± 9.02 | 0.190 |
| BMI | 27.24 ± 4.28 | 31.25 ± 5.05 | < 0.001 |
| SBPa | 119.51 ± 17.07 | 121.57 ± 18.48 | 0.004 |
| DBP | 78.08 ± 13.09 | 79.34 ± 13.01 | 0.004 |
| DMa (%) (95% CI) | 13.8% (12.3% - 15.3%) | 26.1% (23.8% - 28.4%) | < 0.001 |
| Smokinga (%) (95% CI) | 30.2% (28.1% - 32.3%) | 0.85% (0.37% - 1.31%) | < 0.001 |
| ALP (IU/L) | 187.31 ± 80.08 | 180.79 ± 79.10 | 0.022 |
| ALT(IU/L) | 25.25 ± 16.36 | 20.42 ± 13.92 | < 0.001 |
| GGT (IU/L) | 32.31 ± 35.09 | 28.68 ± 23.49 | 0.004 |
| FBS (mg/dL) | 103.03 ± 34.87 | 113.17 ± 48.68 | < 0.001 |
| TG (mg/dL) | 155.24 ± 100.20 | 159.06 ± 108.58 | 0.308 |
| HDLa (mg/dL) | 42.85 ± 11.63 | 44.69 ± 12.03 | < 0.001 |
| LDL (mg/dL) | 110.17 ± 30.62 | 117.49 ± 31.53 | < 0.001 |
| Total cholesterola (mg/dL) | 187.15 ± 41.14 | 200.59 ± 43.29 | < 0.001 |
| HOMA-IR | 2.16 ± 1.90 | 2.85 ± 2.60 | < 0.001 |
Abbreviations: ALP: Alkaline phosphatase; ALT: alanine aminotransferase; BMI: body mass index; FBS: fasting blood sugar; GGT: gamma glutamyl transferase; HDL: high density lipoprotein; HOMA-IR: homeostasis model assessment insulin resistance; LDL: low density lipoprotein; SBP: systolic blood pressure; DBP: diastolic blood pressure; DM: diabetes mellitus; TG: triglyceride.
athe variables utilized in CVD risk assessment tools.
4.1. Univariate Logistic Regression Analyses
According to the results of the univariate analyses using the ACC/AHA tool, ALP was significantly associated with ≥ 7.5%, ≥ 10%, and ≥ 20% 10-year CVD risks in both men and women (P < 0.05). Applying the Framingham tool, there was a significant positive association between ALP and 10-year CVD risks in both genders, except a ≥ 20% 10-y CVD risk in men. GGT did not show any association with the 10-year CVD risk in men, however, there was a significant association between GGT and a ≥ 20% 10-year CVD risk in women. Using the Framingham tool, alanine aminotransferase (ALT) was inversely associated with 10-year CVD risks only in men. Applying the Framingham tool, all p-values, except those for a ≥ 20% 10-year CVD risk, were < 0.001. More details are presented in Table 2.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Wald | OR (95% CI) | P Value | Wald | OR (95% CI) | P Value | |
| ACC/AHA ≥ 7.5% | ||||||
| ALP | 6.90 | 1.002 (1.001 - 1.003) | 0.009 | 69.77 | 1.007 (1.005 - 1.009) | < 0.001 |
| ALT | 20.63 | 0.985 (0.979 - 0.992) | < 0.001 | 0.016 | 0.999 (0.990 - 1.009) | 0.899 |
| GGT | 1.19 | 1.002 (0.998 - 1.005) | 0.275 | 11.64 | 1.011 (1.005 - 1.017) | 0.001 |
| ACC/AHA ≥ 10% | ||||||
| ALP | 4.06 | 1.001 (1.000 - 1.002) | 0.044 | 53.83 | 1.007 (1.005 - 1.009) | 0.044 |
| ALT | 20.29 | 0.984 (0.978 - 0.991) | < 0.001 | 0.005 | 1.000 (0.990 - 1.011) | 0.943 |
| GGT | 0.338 | 1.001 (0.998 - 1.004) | 0.561 | 11.09 | 1.010 (1.004 - 1.017) | 0.001 |
| ACC/AHA ≥ 20% | ||||||
| ALP | 4.85 | 1.002 (1.000 - 1.003) | 0.028 | 11.92 | 1.005 (1.002 - 1.008) | 0.001 |
| ALT | 12.91 | 0.983 (0.974 - 0.992) | < 0.001 | 0.10 | 0.997 (0.976 - 1.018) | 0.752 |
| GGT | 2.14 | 1.002 (0.999 - 1.006) | 0.144 | 0.676 | 1.004 (0.995 - 1.013) | 0.411 |
| Framingham ≥ 10% | ||||||
| ALP | 8.91 | 1.002 (1.001 - 1.003) | 0.003 | 57.00 | 1.007 (1.005 - 1.008) | < 0.001 |
| ALT | 13.66 | 0.988 (0.982 - 0.994) | < 0.001 | 0.923 | 1.004 (0.996 - 1.013) | 0.337 |
| GGT | 1.98 | 1.003 (0.999 - 1.006) | 0.159 | 21.18 | 1.017 (1.010 - 1.025) | < 0.001 |
| Framingham ≥ 20% | ||||||
| ALP | 3.24 | 1.001 (1.000 - 1.003) | 0.072 | 20.58 | 1.006 (1.004 - 1.009) | < 0.001 |
| ALT | 6.48 | 0.989 (0.981 - 0.998) | 0.011 | 0.211 | 1.003 (0.989 - 1.019) | 0.646 |
| GGT | 3.23 | 1.003 (1.000 - 1.006) | 0.068 | 10.94 | 1.011 (1.005 - 1.018) | 0.001 |
Abbreviations: ACC/AHA: American college of cardiology/American heart association; ALP: alkaline phosphatase; ALT: alanine aminotransferase; CI: confidence interval; GGT: gamma glutamyl transferase; OR: odd ratio.
4.2. Multivariate Regression Analyses
Applying the ACC/AHA tool, the multivariate analysis showed that ALP was significantly associated with a ≥ 7.5% 10-year CVD risk (P = 0.003 in men and P < 0.001 in women), ≥ 10% 10-y CVD risk (P = 0.005 in men, and P < 0.001 in women), and ≥ 20% 10-year CVD risk (P < 0.001 in men and P < 0.044 in women). Applying the Framingham approach, a significant positive association was detected between ALP and ≥ 10% as well as ≥ 20% 10-year CVD risks (all P < 0.001). No significant association was detected between 10-year CVD risks and GGT. ALT showed a significant inverse association with 10-year CVD risks based on all the risk levels applying both tools in men (all P < 0.001). In women, this association was significant only for ≥ 7.5% and ≥ 10% 10-year risks using the ACC/AHA (P = 0.018) and Framingham tools (P = 0.028), respectively. More details are presented in Table 3.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Wald | OR (95% CI) | P Value | Wald | OR (95% CI) | P Value | |
| ACC/AHA ≥ 7.5% | ||||||
| ALP | 8.93 | 1.003 (1.001 - 1.005) | 0.003 | 54.34 | 1.011 (1.008 - 1.014) | < 0.001 |
| ALT | 49.34 | 0.962 (0.952 - 0.972) | < 0.001 | 5.59 | 0.981 (0.965 - 0.997) | 0.018 |
| GGT | 2.49 | 1.004 (0.999 - 1.010) | 0.114 | 0.487 | 1.003 (0.995 - 1.011) | 0.485 |
| ACC/AHA ≥ 10% | ||||||
| ALP | 7.74 | 1.003 (1.001 - 1.005) | 0.005 | 37.14 | 1.009 (1.006 - 1.012) | < 0.001 |
| ALT | 41.66 | 0.965 (0.955 - 0.975) | < 0.001 | 3.27 | 0.983 (0.965 - 1.001) | 0.071 |
| GGT | 0.428 | 1.002 (0.997 - 1.006) | 0.513 | 0.392 | 1.003 (0.995 - 1.011) | 0.531 |
| ACC/AHA ≥ 20% | ||||||
| ALP | 13.60 | 1.004 (1.002 - 1.006) | < 0.001 | 4.06 | 1.005 (1.001 - 1.009) | 0.044 |
| ALT | 30.57 | 0.958 (0.944 - 0973) | < 0.001 | 0.825 | 0.985 (0.955 - 1.017) | 0.364 |
| GGT | 0.262 | 1.001 (0.996 - 1.007) | 0.609 | 0.227 | 0.997 (0.983 - 1.011) | 0.634 |
| Framingham ≥ 10% | ||||||
| ALP | 16.31 | 1.004 (1.002 - 1.007) | < 0.001 | 37.95 | 1.010 (1.007 - 1.014) | < 0.001 |
| ALT | 43.14 | 0.964 (0.954 - 0.975) | < 0.001 | 4.86 | 0.979 (0.960 - 0.998) | 0.028 |
| GGT | 1.06 | 1.003 (0.997 - 1.008) | 0.303 | 2.83 | 1.008 (0.999 - 1.017) | 0.092 |
| Framingham ≥ 20% | ||||||
| ALP | 12.26 | 1.004 (1.002 - 1.006) | < 0.001 | 16.26 | 1.009 (1.005 - 1.014) | < 0.001 |
| ALT | 21.47 | 0.968 (0.954 - 0.981) | < 0.001 | 1.34 | 0.982 (0.951 - 1.013) | 0.248 |
| GGT | 0.104 | 1.001 (0.996 - 1.006) | 0.747 | 1.142 | 1.005 (0.996 - 1.015) | 0.285 |
Abbreviations: ACC/AHA, American college of cardiology/American heart association; ALP, alkaline phosphatase; ALT, alanine aminotransferase; CI, confidence interval; GGT, gamma glutamyl transferase; OR, odd ratio.
5. Discussion
The present study revealed a positive association between the liver enzymes GGT and ALP and 10-year CVD risk. However, ALT showed an inverse relationship with 10-year CVD risks. The present study used FRS and ACC/AHA instruments to predict 10-year CVD risks. According to a previous study, the FRS accurately predicted 10-year CVD risks in patients with NAFLD (11). Based on the findings of the present study, GGT was positively associated only with higher levels of 10-year CVD risks in women, whereas ALP was positively associated with various levels of 10-year CVD risks in both men and women. Furthermore, only ALP showed a positive association with 10-year CVD risks independent of the effects of other potential predictors. In the present study, the associations between ALP and CVD risks were stronger in women than in men. In women, particularly after menopause, serum levels of ALP increase and CVD risks dramatically increase. Thus, the strong association between ALP and CVD risks in women could partly be attributed to the elevation in ALP levels in the postmenopausal period (14).
In agreement with the findings of the current study, a meta-analysis of 29 cohort studies containing aggregate data on over 1.23 million participants and 20,406 cardiovascular outcomes showed that baseline levels of GGT and ALP were positively associated with CVD risks in a log-linear fashion (7). The same study reported that AST showed no association with CVD and that ALT was inversely associated with CVD (7). Ford et al. also found an inverse relationship between ALT levels in the normal range and adverse cardiovascular and noncardiovascular outcomes (15). Although the present study revealed an inverse association between ALT and 10-year CVD risks in men, the results for women based on the applied tools and risk levels were inconsistent. The inverse association between ALT and CVD risks may be partly attributable to the confounding effects of age. As age is a key variable in estimates of 10-year CVD risks, it was not entered in the multivariate models in the present study. Previous research demonstrated that increased age critically increased 10-year CVD risks and that there was an inverse relationship between age and liver enzymes, particularly ALT in older adults (16, 17).
In the present study, the inverse association between ALT and 10-year CVD risks was stronger in men than in women. The consumption of alcohol by men may be much higher than women, particularly in Iran. Consequently, higher levels of enzymes, particularly ALT, can be expected in men due to the effects of alcohol (18). Alcohol has also been suggested to have a protective effect against CVD (19). These issues together may explain the stronger inverse association between ALT levels and CVD risks in men than in women. However, excess alcohol consumption does not have a protective effect against CVD risks and can lead to an increase in CVDs, with epidemiological data pointing to U- or even J- shaped relationships between alcohol intake and the incidence of CVDs (20-24).
The strengths of the present study included its use of 2 tools to calculate the outcome variable. Considering different levels of the outcome variable (10-y CVD risks of ≥ 7.5%, ≥ 10%, and ≥ 20%) in different models helped to ensure the accuracy of the relationships observed between the predictor variables and outcome variable (10-year CVD risk) in the present study. The use of 10-year CVD risk predictions can also aid studies of the association between liver enzymes and cardiovascular events during the next decade.
The main limitation of this study was its cross-sectional design, which is not optimal for demonstrating a cause and effect relationship. However, our aim was not to declare a cause and effect relationship, but to show that whether an association exists between 10-year CVD risks and abnormal values of liver enzymes.
5.1. Conclusion
The present study confirmed a positive relationship between 10-year CVD risks and liver enzymes (GGT and ALP), as well as an inverse association with ALT using the ACC/AHA and Framingham risk-assessment tools. However, GGT did not show any association with 10-year CVD risks independent of the effects of other potential predictors.