1. Background
Vaccination is a most successful medical approach against a variety of infectious diseases (1-3). Hepatitis B virus (HBV) is one of the most challenging infections of liver which caused acute and chronic liver disease (4-7). Although an effective preventive vaccine against HBV has been used globally, still this infection is considered as a leading cause of death worldwide. Most vaccines consist of 2 important parts: antigens and adjuvants (1). The word adjuvant is derived from the Latin word “Adjuvare” which means aid or help (1, 8-13). The rational of using adjuvants in the formulation of vaccines is to increase the immunogenicity of antigens. Recent success of new adjuvants dragged the researchers’ attention to the importance of this agent in the formulation of new vaccines (14, 15). However, humoral immunity has an important role in immunizing a person against viral infections by neutralizing virus and inhibiting the entrance to cells. In the course of chronic HBV infection, cellular immunity can help the body to recover better.
Although many adjuvants have been discovered in the laboratories and through the researches, a small number of them can enter to human clinical trials and obtain approval for human vaccines. Both adjuvant activity and the likely side effects are important factors in selecting an adjuvant for human vaccination. The dominance of Th1 or Th2 immune response has a crucial role in the efficacy of different vaccines. Many adjuvants can shift the immune response to one of the mentioned categories. For instance, vaccination against intracellular infection requires strong Th1 responses, and Freund’s adjuvant can provide it, but the major obstacle is that it is not approved for human use. One of the best food and drug administration (FDA) approved vaccine adjuvants is aluminum salt (Alum), which mostly enhances Th2-specific responses (16). Currently, the standard HBV vaccine contains 20 microgram of hepatitis B surface antigen (HBsAg) in combination with 0.5 milligram of aluminum salt as an adjuvant (17). Although Fendrix vaccine benefits from AS04 adjuvant (18, 19), it is not affordable in many countries due to its high cost.
Naloxone is an antagonist of morphine-like receptors that is commonly used to reduce the side effects of drug abuse such as respiratory depression (20, 21). Several studies have shown that naloxone, as an opioid receptors antagonist, has adjuvant effect. However, the mechanism of immunostimulating effect of naloxone is poorly understood. Fortunately, naloxone is a well-tolerated drug considering toxicity and adverse effects, and it is also FDA approved (16, 20, 22, 23). During the last decade, many studies have used this agent as adjuvant in the formulation of different vaccines. They showed that naloxone can induce TH1 pattern of immunity and increase the IFN-γ level (14, 16, 22, 24-26).
In the present study, we aimed at studying the immunostimulating properties of this naloxone in the context of HBsAg alum- based vaccine and comparing the results with Fendrix vaccine, which is known for its potential to trigger the Th1 immune responses. Moreover, in this study, we evaluated the immune response of immunized mice with naloxone/alum mixture and compared the response to Fendrix vaccine.
2. Objectives
In the present study, we aimed at evaluating the immune response of immunized mice with naloxone/alum mixture and comparing the response to Fendrix vaccine. Although the dose of 6 mg/kg of naloxone has previously been demonstrated as an optimum dose for its adjuvant effect on different vaccine formulations (22, 27), due to some contradictory observations, in the current research, the panel of 3 doses (one lower and one higher than 6 mg/kg) was investigated to study the effect of different doses of naloxone on different aspects of immune responses.
3. Methods
3.1. HBV Vaccines and HBsAg
Conventional HBV vaccine (HBsAg formulated in alum adjuvant) and HBsAg, supplied by Pasteur Institute of Iran (Karaj, Iran), were stored at 4°C until use. Also, Fendrix vaccine was purchased from Glaxo Smith Kline company (GSK, USA) for immunization.
3.2. Experimental Animals
For the experiment, 6 to 8 weeks old female inbred Balb/c mice were purchased from pasteur institute of Iran (Karaj, Iran). Mice were housed for 7 days prior to the experiments and given free access to food and water and maintained in standard conditions (light/dark cycle (12 hrs/12 hrs) and 18 to 20°C temperature. All experiments were in accordance with the animal care and use protocol of pasteur institute of Iran.
3.3. Immunization and Study Design
Experimental groups included female Balb/c mice (n = 76), which were divided into 17 groups. Balb/c mice were immunized with 5 µg of vaccines, 3 times on days 0, 14, and 28 subcutaneously. Groups 1 to 17 were administered as follow:
| Group Number | Injection Formula | Dose | Rout of Injection |
|---|---|---|---|
| 1 | Fendrix | 5 µg | subcutaneously |
| 2 | HBsAg + Alum + Naloxone 3 mg per kg | 5 µg | subcutaneously |
| 3 | HBsAg + Alum + Naloxone 6 mg per kg | 5 µg | subcutaneously |
| 4 | HBsAg + Alum + Naloxone 10 mg per kg | 5 µg | subcutaneously |
| 5 | HBsAg + Alum ( conventional vaccine ) | 5 µg | subcutaneously |
| 6 | HBsAg (Unadjuvanted) | 5 µg | subcutaneously |
| 7 | HBsAg + Naloxone 3 mg per kg | 5 µg | subcutaneously |
| 8 | HBsAg + Naloxone 6 mg per kg | 5 µg | subcutaneously |
| 9 | HBsAg + Naloxone 10 mg per kg | 5 µg | subcutaneously |
| 10 | Alum + Naloxone 3 mg per kg | - | subcutaneously |
| 11 | Alum + Naloxone 6 mg per kg | - | subcutaneously |
| 12 | Alum + Naloxone 10 mg per kg | - | subcutaneously |
| 13 | Naloxone 3 mg per kg | - | subcutaneously |
| 14 | Naloxone 6 mg per kg | - | subcutaneously |
| 15 | Naloxone 10 mg per kg | - | subcutaneously |
| 16 | PBS | - | subcutaneously |
| 17 | Alum | - | subcutaneously |
Experimental Groups of the Study
3.4. Lymphocyte Proliferation
Two weeks after final immunization, cell suspension was prepared by dissecting the spleen in cold phosphate buffered saline (PBS) containing 2% fetal bovine serum (FBS) under aseptic condition. Red blood cells were lysed by the addition of 5 mL lysis buffer on cell pellets, and single-cell suspension was prepared and adjusted to 3 × 106 cells/mL in RPMI-1640 (Gibco, Germany). Next, suspension was supplemented with 10% FBS, 4 mM L-glutamine, 25 mM 4 - 2-hydroxyethylpiperazine-1 - 2-ethanesulfonic acid (HEPES), 0.1 mM non-essential amino acid, 1 mM sodium pyruvate, 50 µm 2 ME, 100 µg/mL streptomycin, and 100 IU/mL penicillin. Afterwards, 100 µL of suspension comprising of 3 × 105 cells were dispensed in 96-well culture plates (Nunc, Denmark) and stimulated with 5 µg/mL of HBsAg as recall antigen. Phytohemagglutinin A (5 μg/mL; Gibco) was used as a positive control and unstimulated wells were used as negative controls. All experiments were repeated in triplicates. After 72 hours of incubation at 37°C in 5% CO2 humid incubator, cells were pulsed with 20 μL of 5- bromo-2’-deoxyuridine (BrdU) (Roche, Germany) per well, and culture incubation continued for another 24 hours. Immediately, the plates were centrifuged at 300 × g for 10 minutes, and culture medium was removed completely and dried at 60°C for 30 minutes. Later, by the addition of 200 μL of fixation/denaturation buffer, the cells were permeabilized, and 100 μL of anti-BrdU antibody was added to each well for 90 minutes. The plates were washed 6 times with PBS buffer, and tetra-methyl benzidine (TMB) solution was added to each well and the reaction was continued for 10 minutes and finally stopped by adding 100 μL of 2N H2SO4. Absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader at 450/630 nm.
3.5. Quantitative IL-4 and IFN-γ ELISA
Two weeks after the last immunization, the total number of 3 × 106 spleen cells per milliliter were resuspended in complete RPMI 1640, placed in each well of 24-well plate and stimulated with 5 μg/mL of HBsAg for 72 hours at 37°C in 5% CO2. The supernatants were then collected and assessed for IFN-γ and IL-4 cytokines using commercial ELISA Kit (Quantikine, R and D Systems, US) according to the manufacturer’s manual.
3.6. ELISA of Specific Total Antibodies and IgG1, IgG2a Isotypes
Specific total antibodies were evaluated using an optimized indirect ELISA method. Briefly, 100μL of HBsAg (5 µg/mL) in PBS were coated in 96 well ELISA Maxisorp plates (Nunc, Naperville, IL), followed by overnight incubation at 4°C. The wells were washed 3 times with PBS containing 0.05% Tween 20 (washing buffer) and blocked for 1 hour at 37°C with 5% skimmed milk in PBS buffer (blocking buffer). Serial dilutions of sera at 1/50 to 1/6400 were prepared in 1% phosphate buffered saline with bovine serum albumin (PBS-BSA). After washing the wells, 100 µL of each dilution was added to each well in triplicate and incubated at 37°C for 2 hours. Then, the wells were washed 5 times with washing buffer and incubated for 2 hours at 37°C. After incubation, the wells were washed 5 times with washing buffer and 100µL of 1/10000 dilution of anti-mouse conjugated to horseradish peroxidase (HRP) (Sigma, USA) in phosphate buffered saline with 1% bovine serum albumin (PBS-BSA) added and incubated for 2 hours at 37°C. Later, the wells were washed 5 times with washing buffer and 100 µL of TMB substrate was added and incubated for 30 minutes in a dark place. The reaction was stopped using 100 μL of 2N H2SO4 and color density was measured at A450/630 nm with ELISA plate reader. To detect specific IgG1 and IgG2a subclasses, goat anti- mouse IgG1 and IgG2a secondary antibodies (Sigma, USA) were used.
3.7. Statistical Analysis
Graph pad prism V6.01 was used for statistical analysis, and Mann Whitney U test was used to compare the statistical significance between the experimental groups. Values of P < 0.05 were considered to represent statistically significant differences. Results are presented as Mean ± S.D.
4. Results
4.1. Proliferation Assay
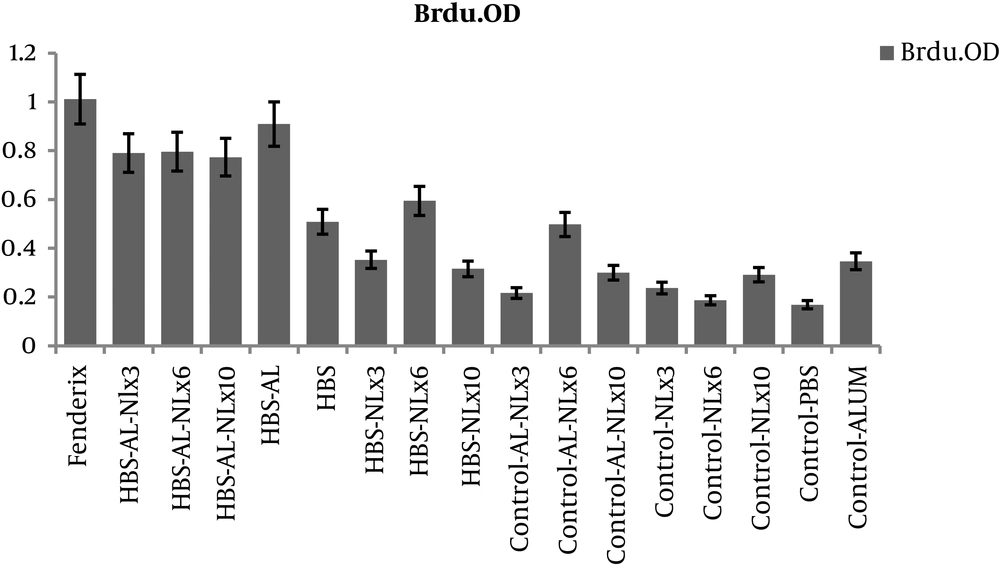
The results of BrdU revealed that proliferation response in all test groups were higher than controls (P < 0.05). However, no significant difference was found between HBsAg-alum formulated vaccine and naloxone/alum mixture formulated vaccine (P > 0.05) (Figure 1).
4.2. Cytokines Assay in Spleen Cell Culture
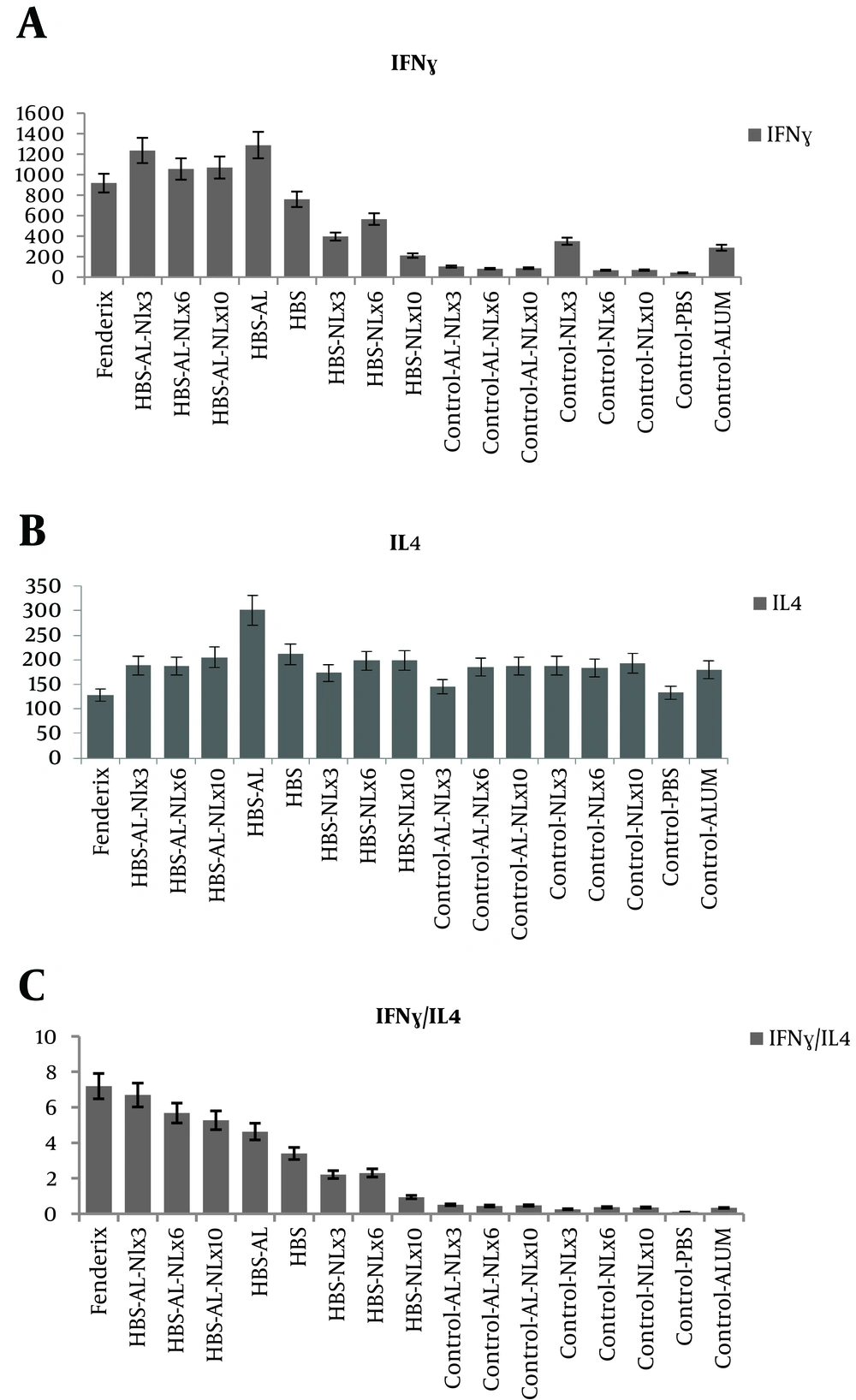
The results showed that IFN-γ significantly increased in test groups compared to control groups. However, there were no significant difference between alum and naloxone/alum formulation in IFN-γ with respect to the same level of IL-4 in all test and control groups of this study. (Figure 2A and 2B). Our findings demonstrated that IFN-γ/IL-4 ratio in naloxone/alum formulated vaccine was higher than HBsAg alum vaccine (Figure 2C). This was more considerable in the dose of 3 mg per kg of naloxone.
A, Effect of Administering Naloxone/Alum Mixture on IFN-γ Production. Two Weeks After the Final Immunization, the Spleens of Individual Mice Were Removed and IFN-γ Production Was Measured and Compared Between Experimental Groups; B, Effect of Administering Naloxone/Alum Mixture on IL-4 Production. Two Weeks After the Final Immunization, the Spleens of Individual Mice Were Removed and IL-4 Production Was Measured and Compared Between Experimental Groups; C, Evaluating IFN-γ/IL-4 Cytokine Level in Experimental Groups. Data Are Shown As Mean ± SD
4.3. Humoral Immune Response and Isotyping
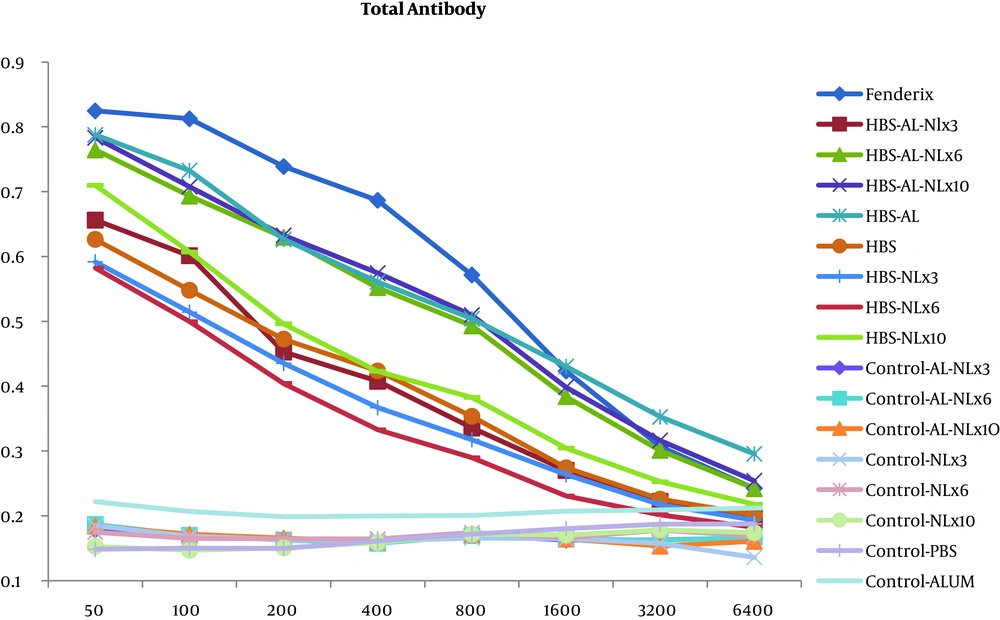
Considering the results of the total antibody, Fendrix vaccine was more successful in the induction of antibody response and the capacity of humoral immunity in consequence of HBV (Figure 3).
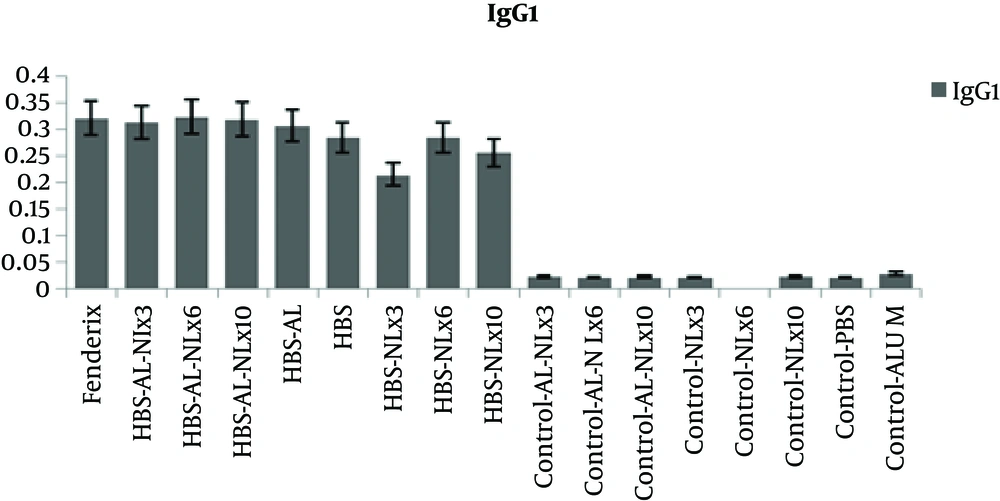
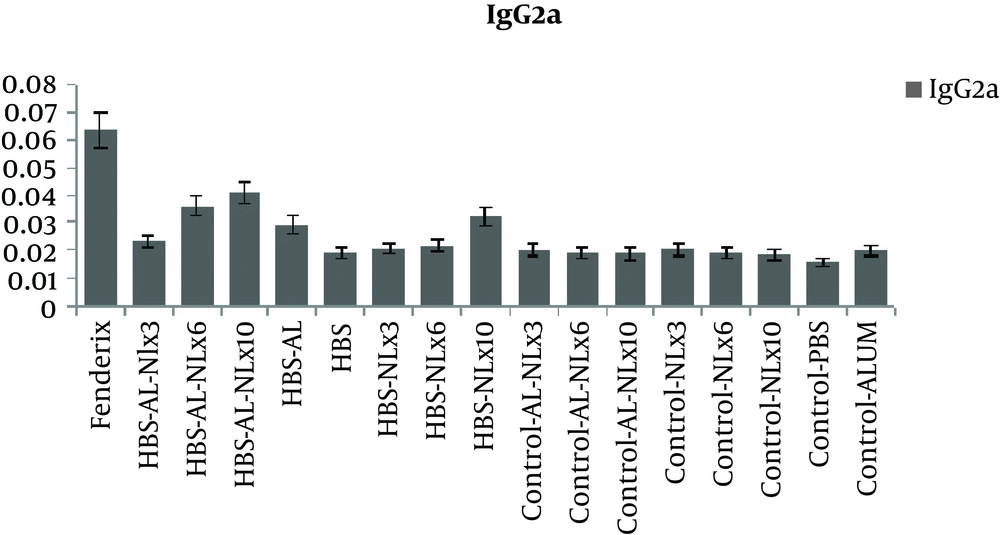
The level of antibody isotypes (Figure 4 and 5) showed that IgG1 in all test groups was in the same level, but Fendrix and alum/naloxone 10 mg /kg based vaccine were more successful in inducing higher level of IgG2a, that is Th1 marker.
Evaluating IgG2a Antibody Level of the Candidate Vaccine. Effect of Administering Naloxone/Alum Mixture on Antibody Response. The Sera Obtained Two Weeks After the Last Immunization, From All of the Mouse Groups, Were Screened for the Presence of IgG2a Against HBsAg. Data Are Shown As Mean ± SD
5. Discussion
It is well accepted that an appropriate HBV vaccine should have the ability to induce cellular and humoral immunity. In the present work, we used naloxone as opioid receptor antagonist in the formulation of alum- based HBV vaccine to induce higher level of Th1 cytokine IFN-γ, which can better help B cells to produce antibody.
The role of adjuvants in vaccine formulation is to modulate and increase the vaccine specific immune response. Although several adjuvants are being used in clinical trials, alum is the only FDA approved adjuvant for human vaccines. Alum shifts the immune responses towards Th2 immunity (28, 29), which is responsible for antibody production. With regards to the appropriate potential of alum- based vaccines to boost humoral immunity, most of them have not been successful to induce efficient response against intracellular pathogens. One reason for this phenomenon may be due to the lack of proper induction of IFN-γ (30).
Naloxone is routinely used for rapid and safe reverse opioid-induced respiratory depression. Opioid receptors, particularly κ and δ opioid receptors, are expressed in immune cells. Opioid receptors can modulate both innate and acquired immune responses (31); μ opioid receptor agonists can have anti-proliferative effects of delta opioid receptor (DOR) agonists, inhibiting IFN-γ production (16). Recent evidences suggest that naloxone as an antagonist for these receptors may induce a pro-inflammatory milieu by increasing the release of local pro-inflammatory neuropeptides such as substance P from the nerve fibers, or by a direct effect on innate immune cells including monocytes, macrophages, and dendritic cells. The off label use of naloxone is to boost the immune response and stimulate the production of pro- inflammatory cytokines. Antigens in this pro-inflammatory milieu can promote specific immunity toward cell-mediated immunity. On the other hand, neuropeptides may promote the maturation and migration of local antigen-presenting cells to the draining lymph nodes and trigger Th1 paradigm of immune responses (31).
Previous studies (Sacerdote in 1998 and 2000) revealed that naloxone as an opioid antagonist is able to trigger the immune response to the Th1 pattern, and is also able to strengthen immune responses after vaccination (24, 25). Jamali et al. observed that naloxone can increase the levels of IFN-γ cytokine and cellular immune responses against herpes simplex virus infection, and hypothesized the possibility of adjuvant effect of naloxone on HSV-I vaccine (27). Further studies on HSV-1 and Listeria monocytogenes in 2009 and 2010, endorsed the adjuvant effect of this medicine to strengthen the level of cellular immune responses (16, 23).
Results of the current study indicated that vaccination with HBsAg plus alum and naloxone (3 mg per kg) had an impact on the ratio of IFN-γ/IL-4 and caused an increase in the level of IFN-γ, indicating the induction of Th1 immunity. It can be deduced that existence of naloxone in HBsAg-alum vaccine formulation can cause more reinforcement of cellular immune response. However, the results of total antibody revealed no significant difference between HBsAg-alum group and HBsAg-alum-naloxone groups. The likely reason for the use of naloxone as an adjuvant in the formulation of different vaccines by other studies (16, 22, 23, 27, 32) could be the nature of HBsAg that by itself has the ability to stimulate humoral immunity. As it was already expected, Fendrix vaccine was more successful in the induction of higher level of humoral immunity. Although, naloxone along with other antigens, can significantly increase the capacity of humoral immunity, this result was not entirely achieved in the current study. One predicted reason for this difference is related to the physicochemical properties of adjuvants and antigens. The bond form of any adjuvant is highly effective in boosting the immune response. In Fendrix vaccine, MPL has a physical bond with alum and acts as an adjuvant element to stimulate toll like receptors where antigens are in touch with antigen presenting cells. However, in our formulation, naloxone was admixed to the alum and they may not have physical attachment. In fact, in the mixture of alum and naloxone as an immune potentiator, the lack of physical attachment may affect the quality of immune responses. Therefore, it is highly suggested to study the effect of bond form of naloxone to alum in the further studies.
Although the lymphocytes proliferation pattern in the naloxone- based vaccine groups were higher than control groups, Fendrix vaccine induced maximum level of proliferation. However, increase in lymphocyte proliferation alone is not an absolute index for judging the type of immune response and IFN-γ / IL-4 ratio is more valuable and accurate index, which in our research, showed the benefit of naloxone in the reinforcement of Th1 immunity.
New scientific findings show that the best pattern of immune response to control viral infection is the mixture of Th1 and Th2 responses. In fact, Th1 by eradicating the virally infected cells and Th2 by producing antibodies which neutralize the viruses and prevents the cellular entrance step, together can help the body to be cleared from infection. In any vaccine, particularly hepatitis B vaccine, triggering the Th2 immunity which produces the antibody is not the only desirable response. On the other hand, as the studies on individuals that show no response to vaccine have shown, the stimulation of IFN-γ production and increase in the level of this important Th1 cytokine in this category can cause seroconversion and better immunization outcome (33, 34).
It seems that in the non-responder individuals, B cell may not receive adequate help from T helper cells, which can be due to lack of proper stimulation of T cells. Therefore, the increase in the level of IFN-γ in our research may indicate better T-cell stimulation, better B cells function, and antibody production. The other finding of the current work which may show the ability of naloxone in vaccine formulation to induce the better immunization response, is the increase in the level of IgG2a. IgG2a is produced due to the activation of Th1 cells or activation of cellular immunity against the intended antigens. Although only the dose of 10 mg/kg of naloxone caused a considerable increase in the level of this isotype of antibodies, the ability of naloxone to switch the antibody response due to the enhancing effect on the production of IFN-γ, compared to IL-4, can be easily observed.
5.1. Conclusions
Taken together, the results of IgG2a and IFN-γ/IL-4 ratio of naloxone- based vaccines in the present work were considerable and in some doses was as same as Fenderix. However, considering the results, we are more hopeful to improve the efficacy of alum- based vaccine. However, to reach a better understanding of immunomodulatory effect of naloxone and investigate all aspects of corresponding immune response, it is highly suggested to use naloxone in combination with other adjuvants or in physically attached forms with alum.