1. Background
Liver injury is a prevalent form of organ damage in patients with SARS-CoV-2. It is estimated that 14% to 53% of patients with COVID-19 develop abnormal liver function tests (1). The mechanisms of liver damage in COVID-19 are under investigation and appear to have multifactorial etiologies. Liver injury may present with subclinical elevation of aminotransferase levels or fulminant hepatic failure (2, 3). Therefore, SARS-CoV-2-related liver injury has a spectrum of histopathological features. Autopsy studies have demonstrated steatosis, inflammation, vascular thrombosis, and hepatocyte necrosis in deceased patients with COVID-19 (4-7). According to recent pathology investigations, patients with COVID-19 had a greater rate of steatosis and lobular inflammation than those with other liver diseases. High-dose steroid administration contributes to the occurrence of steatosis (6). Liver injury is associated with prolonged length of stay (LOS) in hospital, higher rates of ICU admission, and increased mortality rates (8). Critically ill patients with COVID-19 exhibit increased hepatic failure, suggesting that liver damage is correlated with disease severity (9). Additionally, pre-existing liver disorders, including non-alcoholic fatty liver disease (NAFLD) and chronic viral hepatitis, may exacerbate the risk of liver damage (10). SARS-CoV-2-associated liver damage contributes to unfavorable outcomes in patients with COVID-19.
2. Objectives
In the present study, we aimed to evaluate liver histopathology and its clinical correlation in a series of patients who died of COVID-19.
3. Methods
We conducted a case series study of deceased patients with COVID-19 in Tehran, Iran, between January and May 2021. Subjects were selected through conventional sampling after obtaining written informed consent from their first-degree relatives. One of the authors, wearing protective equipment, including an N95 mask, gown, gloves, and face shield, performed the liver biopsy in a negative pressure room. The procedure was performed using a 16-gauge Jamshidi needle through the 12th right intercostal space. The samples were fixed in formalin and transferred to the pathology department. Subsequently, 10 µm sections were prepared from the samples using a microtome, stained with hematoxylin-eosin, and mounted on slides. Each slide was independently examined by two pathologists, and the final report was compiled through collaboration between the attending physician and pathologists. Additionally, demographic characteristics, underlying conditions, clinical symptoms, and paraclinical findings, including blood cell counts, inflammatory biomarkers, liver function tests, and histological findings, were extracted from their registries. Fisher’s exact test and Student’s t-test were used for data analysis. This study is part of a multi-organ autopsy study in deceased patients with COVID-19, approved by the Ethics Committee of the AJA University of Medical Sciences (IR.AJAUMS.REC.1399.030).
4. Results
During the study period, 12 deceased COVID-19 patients underwent liver biopsies. Of these, three were female and nine were male. The mean age of the patients was 73 ± 14.3 years, the mean Body Mass Index (BMI) was 26.6 ± 4, and the mean LOS was 12.3 ± 3.3 days. The frequencies of underlying diseases included hypertension (HTN) in 11 patients (91.7%), ischemic heart disease (IHD) in eight patients (66.7%), diabetes mellitus (DM) in five patients (41.7%), and chronic renal failure (CRF) in three patients (25%). The most common symptoms on admission were dyspnea in 11 patients (91.7%), cough in nine patients (75%), fever in seven patients (58.3%), malaise, and decreased level of consciousness in six patients (50%). The most common clinical cause of death was adult respiratory distress syndrome (ARDS), which occurred in eight patients (66.7%).
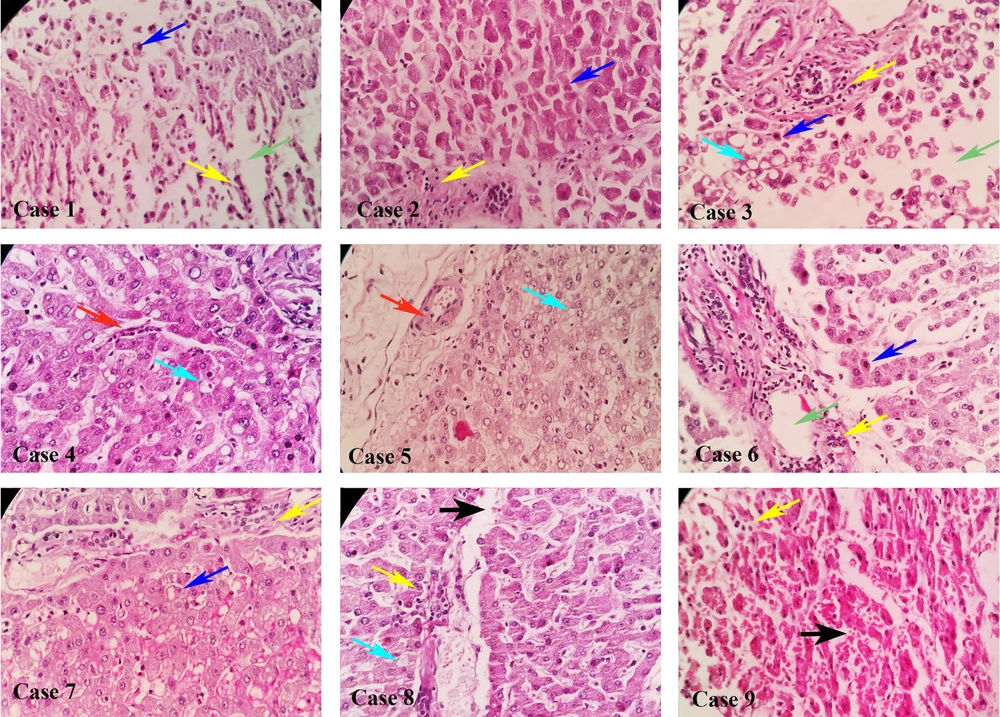
Table 1 presents the demographic characteristics, clinical symptoms, and laboratory findings of patients on admission. Histopathological examination revealed portal and periportal inflammation as the predominant findings in nine patients (75%), followed by microvesicular steatosis in four patients (33.3%) and interstitial edema in three patients (25%) (Figure 1). Hepatic inflammation was classified as minimal to mild in four individuals and moderate to severe in eight patients according to the NAFLD activity score (NAS score). The Student’s t-test (Table 2) did not reveal any correlation between the NAS score and age, BMI, LOS, C-reactive protein (CRP), lactate dehydrogenase (LDH), and alanine transaminase (ALT) levels. Additionally, Fisher's exact test did not reveal any correlation between NAS score and sex, DM, CRF, or etiology of mortality (Table 3). Patients with steatosis exhibited a higher BMI than those without steatosis (30.2 ± 3.6 vs. 24.7 ± 2.8, respectively; P = 0.014).
| Cases | Age | Sex | BMI (kg/m2) | LOS | ALC | ANC | Hb | PLT (× 103 U/L) | CRP (mg/L) | LDH (< 450 U/L) | ALT (< 40 IU/L) | PMH | Clinical Cause of Death | Hepatic Inflammation by NAS Score | Liver Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | 24 | 7 | 873 | 8342 | 14.9 | 143 | 92 | 765 | 13 | HTN, CRF | PTE | Moderate activity, 3 points | Portal and periportal mixed inflammation, ballooning degeneration, edema |
| 2 | 79 | F | 29 | 12 | 640 | 6720 | 12.1 | 317 | 2 | 310 | 16 | HTN, DM | ARDS | Mild activity, 2 points | Portal and periportal mixed inflammation, ballooning degeneration |
| 3 | 70 | F | 27 | 17 | 666 | 2960 | 14.4 | 136 | 65 | 753 | 78 | HTN | PTE | Moderate activity, 5 points | Portal and periportal mixed inflammation , microvesicolar steatosis, focal necrosis, ballooning degeneration, edema |
| 4 | 35 | M | 35 | 8 | 1910 | 16808 | 11.4 | 321 | 64 | 844 | 84 | HTN, IHD, DM | ARDS | Moderate activity, 3 points | Mild inflammation, microvesicolar steatosis |
| 5 | 79 | M | 31 | 10 | 770 | 4510 | 14.8 | 115 | 43 | 1573 | 13 | HTN, IHD | ARDS | Moderate activity, 4 points | Mild inflammation, microvesicolar steatosis |
| 6 | 78 | M | 21 | 12 | 1262 | 10500 | 12.7 | 212 | 54 | 280 | 24 | HTN, CRF | ARDS | Severe activity, 7 points | Portal and periportal mixed inflammation, ballooning degeneration, edema |
| 7 | 93 | M | 24 | 12 | 1221 | 15576 | 13.8 | 162 | 68 | 693 | 33 | None | ARDS | Moderate activity, 4 points | Portal and periportal mixed inflammation, ballooning degeneration |
| 8 | 69 | M | 28 | 10 | 3250 | 20750 | 9.2 | 397 | 66 | 476 | 66 | HTN, IHD | ARDS | Moderate activity, 4 points | Portal and periportal mixed inflammation and hemorrhage, microvesicolar steatosis |
| 9 | 64 | M | 28 | 13 | 1137 | 4152 | 13.8 | 137 | 46 | 827 | 33 | HTN, IHD, DM | PTE | Moderate activity, 4 points | Portal and periportal mixed inflammation, hemorrhage |
| 10 | 75 | M | 24 | 17 | 567 | 5670 | 12.7 | 190 | 74 | 614 | 34 | HTN, IHD | Sepsis and MOF | Normal | Normal |
| 11 | 70 | M | 22 | 16 | 2250 | 12320 | 13.9 | 126 | 72 | 365 | 30 | HTN, IHD, DM, CRF | Sepsis and MOF | Normal | Normal |
| 12 | 86 | M | 26 | 14 | 336 | 2040 | 12.7 | 83 | 58 | 550 | 45 | HTN, IHD, DM | PTE | Normal | Normal |
Abbreviations: BMI, Body Mass Index; LOS, length of stay; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CRP, C-reactive protein; LDH, lactate dehydrogenase; ALT, alanine transaminase; F, female; M, male; HTN, hypertension; CRF, chronic renal failure; DM, diabetes mellitus; IHD, ischemic heart disease; NAS score, non-alcoholic fatty liver disease (NAFLD) activity score.
| Variables | NAS Score | P-Value (Fisher’s Exact Test) | |
|---|---|---|---|
| Normal or Mild Score (n = 4) | Moderate or Severe Score (n = 8) | ||
| Gender | 1.000 | ||
| Male | 3 | 6 | |
| Female | 1 | 2 | |
| DM | 0.081 | ||
| Yes | 4 | 3 | |
| No | 0 | 5 | |
| CRF | 1.000 | ||
| Yes | 1 | 2 | |
| No | 3 | 6 | |
| Death etiology | 0.067 | ||
| ARDS | 1 | 7 | |
| PTE | 1 | 1 | |
| Sepsis | 2 | 0 | |
Abbreviations: NAS score, non-alcoholic fatty liver disease (NAFLD) activity score; DM, diabetes mellitus; CRF, chronic renal failure; ARDS, adult respiratory distress syndrome.
| Variables | NAS Score | P-Value (t-Test) | |
|---|---|---|---|
| Normal or Mild Score (n = 4) | Moderate or Severe Score (n = 8) | ||
| Age | 73 ± 12.5 | 70.7 ± 16.9 | 0.820 |
| BMI | 27 ± 3.9 | 27.2 ± 4.4 | 0.926 |
| LOS | 16.7 ± 22.2 | 5.9 ± 2.1 | 0.180 |
| CRP | 42.5 ± 30.4 | 62.2 ± 15.3 | 0.156 |
| LDH | 416 ± 104 | 776 ± 375 | 0.095 |
| ALT | 26.5 ± 14.1 | 43 ± 28.7 | 0.312 |
Abbreviations: BMI, Body Mass Index; LOS, length of stay; CRP, C-reactive protein; LDH, lactate dehydrogenase; ALT, alanine transaminase; NAS score, non-alcoholic fatty liver disease (NAFLD) activity score.
5. Discussion
Our study is one of the few investigations focused on liver histopathology in patients with COVID-19. The most common histopathological finding was inflammation (75%), followed by steatosis (33.3%). Most patients were classified as having moderate or severe NAS scores, indicating the severity of liver injury in COVID-19. Our findings underscore the importance of liver involvement in patients with severe COVID-19. Furthermore, steatosis was more common in patients with a BMI > 30. Similar studies have reported steatosis, inflammation, and necrosis as primary findings in liver pathology. The primary liver histological characteristics linked to SARS-CoV-2 infection include steatosis, portal and periportal inflammation, vascular thrombosis, and hepatocyte necrosis (4, 5, 9-14). However, we did not observe portal thrombosis in our subjects, which may be attributable to the small sample size or population differences. We also did not find fibrotic changes in our patients. Prolonged liver inflammation may result in hepatic fibrosis in patients with liver diseases (15). We suggest that since only a quarter of our patients exhibited increased transaminases and none had a history of chronic viral hepatitis, along with the acute nature of inflammation triggered by COVID-19, the development of fibrosis seemed unlikely. Although we did not find any correlation between the NAS score and other clinical or paraclinical parameters, this may be related to our limited sample size.
Angiotensin-converting enzyme 2 (ACE2) is a specific receptor for SARS-CoV-2 and is present in several vital organs (16). Autopsy studies have revealed severe inflammation and damage to the lungs, heart, brain, kidney, and liver in a high percentage of patients with severe COVID-19 (17-20). The most common finding of liver injury during SARS-CoV-2 infection is elevated transaminase levels (1, 21, 22). Furthermore, some patients develop cholestasis and elevated alkaline phosphatase and bilirubin levels (23). Acute hepatic failure is a rare condition that develops in severe COVID-19 cases (3, 24). Several mechanisms have been proposed for liver injury during SARS-CoV-2 infection, including direct SARS-CoV-2 invasion (25), immune-mediated injury after cytokine storms (26), hypoxia-related liver injury (27), coagulopathy (28), and drug-induced hepatotoxicity, such as corticosteroids resulting in steatosis, or remdesivir resulting in hepatitis (14, 29). Pre-existing hepatic conditions, advanced age, and severe COVID-19 increase the likelihood of hepatic complications (10).
Previous studies have reported that liver injury in patients with COVID-19 is associated with prolonged LOS, higher rates of ICU admission, and increased mortality rates (8). Although studies have suggested a correlation between liver inflammation and disease severity, we did not find any correlation between the NAS score and CRP levels in our study (9, 14). While additional research is required to definitively ascertain the relationship between liver inflammation and COVID-19-related mortality, existing evidence indicates that hepatic inflammation significantly influences the prognosis and outcome of COVID-19. Despite the absence of an association between the NAS score and the history of DM, CRF, CRP levels, or BMI, this inconsistency may be attributable to the limited sample size of our study.
5.1. Limitations
Our study has several limitations. The principal limitation was the absence of a control group to compare liver pathology in hypoxic-ischemic liver injury with that in viral hepatitis. Another challenge was the absence of prior liver function tests, which affected liver pathology before admission. Finally, our study was conducted with a small subject group, which may limit the generalizability of our findings.
5.2. Conclusions
Our study revealed a high percentage of liver involvement in deceased COVID-19 patients. Therefore, clinicians should monitor liver function in patients with COVID-19, particularly in those with preexisting liver conditions.