1. Background
Esophageal carcinoma is one of the most prevalent forms of cancer globally, with an unfavorable prognosis. The incidence and mortality rates of this cancer vary by region, with approximately 80% of cases occurring in underdeveloped countries (1). Recent statistics indicate a rising incidence of esophageal cancer, particularly in developing countries, with a notable increase in adenocarcinoma cases attributed to obesity and gastroesophageal reflux disease. According to the Global Cancer Observatory, approximately 604,000 new cases were reported in 2020, underscoring the urgent need for effective treatment strategies (2).
The primary treatment for esophageal cancer involves surgical resection, often combined with neoadjuvant or adjuvant therapies such as chemotherapy and radiation. Despite advancements in surgical techniques and perioperative care, managing thrombotic risks during surgery remains critical, leading to the frequent use of low molecular weight heparins (LMWH) to prevent venous thromboembolism (VTE). However, the hepatotoxic potential of LMWH, particularly concerning liver function during the perioperative period, has not been thoroughly investigated (3).
Currently, esophagectomy is an effective method for treating esophageal cancer. However, due to the high failure rate of a single method (i.e., esophagectomy alone), most cases are treated with combination therapy, including neoadjuvant radiotherapy and chemotherapy or perioperative chemotherapy (4). The LMWH are anticoagulants derived from unfractionated heparin through depolymerization, featuring shorter chains of glycosaminoglycans with a molecular weight typically between 2,000 and 8,000 Daltons. Their structure includes sulfated glucosaminoglycans that enhance the activity of antithrombin III, primarily inhibiting factor Xa and, to a lesser extent, factor IIa (thrombin) (5).
The LMWH are widely utilized in clinical settings for the prophylaxis and treatment of VTE, acute coronary syndrome, and are considered safe for use during pregnancy (6). Recent studies have also explored their potential antitumor properties and benefits in postoperative recovery, highlighting their favorable safety profile compared to unfractionated heparin. The LMWH, as an anticoagulant, have been widely used in the perioperative period of esophageal cancer, and low-molecular heparin is easier to administer subcutaneously, with a longer half-life and no need for frequent dosing (7).
The LMWH has emerged as a promising adjunct therapy in the management of esophageal cancer. Traditionally used for its anticoagulant properties, recent studies suggest that LMWH may also possess antitumor effects, potentially improving patient outcomes. Research indicates that LMWH can inhibit tumor growth and metastasis by modulating the tumor microenvironment and enhancing immune responses (8). It is important to mention that drug-induced hepatotoxicity is among the frequently observed adverse reactions to medication in a clinical setting. The symptoms vary from slight liver impairment to sudden liver collapse. Bleeding is the primary side effect of low molecular weight heparin, which is widely utilized as a parenteral anticoagulant (9). However, it is not uncommon to report hepatotoxicity caused by low molecular weight heparin. The research focused on 31 instances of complete removal of esophageal cancer, aiming to analyze the impact of low molecular weight heparin on liver function during the perioperative period of esophageal cancer surgery (10).
2. Objectives
The present study aims to explore the impact of LMWH on liver function in patients undergoing complete esophageal cancer resection, thereby contributing to the development of safer therapeutic strategies for this vulnerable population.
3. Methods
3.1. Objectives
A retrospective analysis was conducted on 31 instances of complete endoscopic radical removal of esophageal cancer that occurred between February 2023 and June 2024. The average age of the patients was 57.32 ± 6.91 years. All individuals met the diagnostic criteria for esophageal carcinoma and had preoperative child grade A liver function. Their clinical data were comprehensive and precise, with no records of drug hypersensitivity, mental illness, or other medical conditions. Moreover, informed consent forms were signed by all patients’ relatives. Exclusion criteria included coagulation dysfunction, serious damage to the function of important organs such as the heart, liver, and kidneys, a history of mental illness, and patients who could not cooperate with the completion of this study.
3.2. Study Group
A total of 31 cases were divided into two groups, namely the LMWH group (n = 21) and the control group (n = 10), using random allocation. Patients in the LMWH group initiated anticoagulant treatment using LMWH 24 hours post-surgery. One patient ceased heparin on the 11th day, 13 patients ceased heparin on the 7th day, and 7 patients continued heparin. Conversely, individuals in the control group did not undergo postoperative anticoagulant therapy with LMWH. If a patient had liver function damage, it was treated with magnesium isoglycyrrhizinate and glutathione, and if the liver function did not significantly improve, low molecular weight heparin was stopped.
3.3. Observation Indicators
3.3.1. Statistical Analysis of Clinical Data of Two Groups of Patients
Clinical data of patients were selected, including gender, height, age, presence or absence of basic diseases (hypertension, diabetes, and coronary heart disease), operation mode (via mediastinum and full-cavity endoscope), ligation of thoracic duct, operation time, blood loss, and postoperative hospitalization time.
3.3.2. Analysis of Liver Function Indexes of Patients in the Two Groups
Collect 5 mL of blood samples from the elbow vein prior to surgery, on the day following surgery, on the third day following surgery, on the fifth day following surgery, on the seventh day following surgery, and on the 11th day following surgery, respectively. Centrifuge the samples at a speed of 3000 rotations per minute for 10 minutes, then collect the liquid above the sediment. Finally, utilize an automatic biochemical analyzer to determine the levels of direct bilirubin, indirect bilirubin, cholinesterase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT).
3.4. Statistical Methods
The data in this study were processed using SPSS 20.0 statistical analysis software (IBM, USA). The measurement data were expressed as "mean ± standard deviation" (± SD), and the comparison between groups was analyzed by one-way ANOVA. Counting data were expressed as percentages (%), and χ2 analysis was used for comparison between groups. A P-value of < 0.05 was considered statistically significant.
4. Results
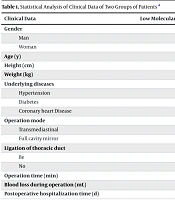
4.1. Statistical Analysis of Clinical Data of Two Groups of Patients
No notable distinctions were observed between the two groups in terms of gender, height, age, weight, underlying conditions (such as hypertension, diabetes, coronary heart disease), surgical approach (mediastinum, full-cavity endoscope), thoracic duct ligation, duration of surgery, amount of blood loss, and length of postoperative hospital stay (P > 0.05) (Table 1).
| Clinical Data | Low Molecular Weight Heparin Group (n = 21) | Control Group (n = 10) | χ2/t-Value | P-Value |
|---|---|---|---|---|
| Gender | 1.001 | 0.105 | ||
| Man | 15 (71.43) | 8 (80.00) | ||
| Woman | 6 (28.57) | 2 (20.00) | ||
| Age (y) | 70.19 ± 6.41 | 69.50 ± 7.15 | 0.473 | 0.648 |
| Height (cm) | 165.95 ± 7.86 | 167.30 ± 8.34 | 0.719 | 0.491 |
| Weight (kg) | 62.30 ± 9.69 | 62.20 ± 6.80 | 0.272 | 0.792 |
| Underlying diseases | 0.135 | 0.905 | ||
| Hypertension | 9 (42.86) | 3 (30.00) | ||
| Diabetes | 2 (9.52) | 2 (20.00) | ||
| Coronary heart Disease | 1 (4.76) | 1 (10.00) | ||
| Operation mode | 1.000 | 1.000 | ||
| Transmediastinal | 0 | 0 | ||
| Full cavity mirror | 21 (100.00) | 10 (100.00) | ||
| Ligation of thoracic duct | 1.000 | 1.000 | ||
| Be | 1 (4.76) | 0 | ||
| No | 20 (95.24) | 10 (100.00) | ||
| Operation time (min) | 132.29 ± 51.93 | 131.00 ± 15.06 | 0.963 | 0.176 |
| Blood loss during operation (mL) | 52.86 ± 4.03 | 40.00 ± 25.82 | 1.281 | 0.232 |
| Postoperative hospitalization time (d) | 11.48 ± 2.38 | 10.70 ± 1.77 | 1.081 | 0.308 |
a Values are expressed as No. (%) or mean ± SD.
4.2. Comparison of Direct Bilirubin Levels Between the Two Groups Before and After Operation
No notable disparity in the levels of direct bilirubin was observed between the two groups prior to surgery, as well as on the first, third, fifth, seventh, and eleventh days post-surgery (P > 0.05). The direct bilirubin level in both groups began to rise on the first day following surgery and subsequently declined by the 11th day post-surgery. This change was statistically significant (P < 0.05) (Table 2).
| Groups | Preoperative | The First Day After Operation | On the Third Day After Operation | On the Fifth Day After Operation | The 7th Day After Operation | On the 11th Postoperative Day | Variance Ratio | P-Value |
|---|---|---|---|---|---|---|---|---|
| Low molecular weight heparin group (n = 21) | 3.65 ± 1.16 | 4.71 ± 2.39 | 5.41 ± 2.09 | 5.77 ± 4.04 | 5.44 ± 4.75 | 4.33 ± 3.04 | 14.421 | 0.001 |
| Control group (n = 10) | 3.48 ± 1.26 | 3.95 ± 1.80 | 3.72 ± 1.17 | 3.89 ± 1.47 | 4.67 ± 1.93 | 4.40 ± 1.73 | 13.978 | 0.001 |
| t-value | 0.204 | 0.379 | 1.375 | 0.981 | 0.045 | 0.228 | - | - |
| P-value | 0.843 | 0.713 | 0.208 | 0.352 | 0.965 | 0.825 | - | - |
a Values are expressed as mean ± SD.
4.3. Comparison of Preoperative and Postoperative Indirect Bilirubin Levels Between the Two Groups
No notable disparity in the level of indirect bilirubin was observed between the two groups prior to surgery, as well as on the first, third, fifth, seventh, and eleventh days post-surgery (P > 0.05). Indirect bilirubin levels in both groups began to rise on the first day following surgery and subsequently declined by the seventh day post-operation. This change was statistically significant (P < 0.05) (Table 3).
| Groups | Preoperative | The First Day After Operation | On the Third Day After Operation | On the Fifth Day After Operation | The 7th Day After Operation | On the 11th Postoperative Day | Variance Ratio | P-Value |
|---|---|---|---|---|---|---|---|---|
| Low molecular weight heparin group (n = 21) | 4.69 ± 2.18 | 5.51 ± 3.60 | 7.49 ± 4.42 | 4.57 ± 3.03 | 3.80 ± 3.00 | 3.22 ± 1.74 | 9.856 | 0.001 |
| Control group (n = 10) | 4.24 ± 2.28 | 4.63 ± 2.76 | 4.38 ± 1.82 | 3.34 ± 1.55 | 3.34 ± 1.49 | 3.80 ± 2.33 | 5.885 | 0.001 |
| t-value | 0.087 | 0.068 | 1.700 | 1.274 | 0.740 | 1.132 | - | - |
| P-value | 0.932 | 0.947 | 0.123 | 0.235 | 0.478 | 0.287 | - | - |
a Values are expressed as mean ± SD.
4.4. Comparison of Cholinesterase Levels Between the Two Groups Before and After Operation
There was no significant difference in cholinesterase levels between the two groups before operation, the first day after operation, the third day after operation, the fifth day after operation, the seventh day after operation and the eleventh day after operation (P > 0.05). The levels of cholinesterase in both groups began to decrease on the 1st postoperative day and to increase on the 7th postoperative day. There was a significant difference between preoperative and postoperative cholinesterase levels (P < 0.05) (Table 4).
| Groups | Preoperative | The First Day After Operation | On the Third Day After Operation | On the Fifth Day After Operation | The 7th Day After Operation | On the 11th Postoperative Day | Variance Ratio | P-Value |
|---|---|---|---|---|---|---|---|---|
| Low molecular weight heparin group (n = 21) | 6499.57 ± 1314.16 | 5793.95 ± 1118.63 | 4928.62 ± 1235.37 | 4810.19 ± 1305.27 | 5071.62 ± 1331.87 | 5303.95 ± 1268.79 | 22.208 | 0.001 |
| Control group (n = 10) | 7351.00 ± 1265.61 | 6788.60 ± 788.77 | 5792.40 ± 1022.30 | 5164.50 ± 863.43 | 5608.90 ± 1086.88 | 6382.50 ± 1234.78 | 18.367 | 0.001 |
| t-value | 0.989 | 1.990 | 1.577 | 0.574 | 0.974 | 1.751 | - | - |
| P-value | 0.348 | 0.078 | 0.149 | 0.580 | 0.356 | 0.114 | - | - |
a Values are expressed as mean ± SD.
4.5. Comparison of Alanine Aminotransferase Levels Between the Two Groups Before and After Operation
There was no notable disparity in the levels of ALT between the two groups prior to surgery, immediately following surgery, five days after surgery, and seven days after surgery (P > 0.05). However, there was a significant contrast in the levels of ALT between the two groups on the third day and the eleventh day after surgery (P < 0.05). Additionally, there was a significant distinction in the levels of ALT between the two groups before and after surgery (P < 0.05) (Table 5).
| Groups | Preoperative | The First Day After Operation | On the Third Day After Operation | On the Fifth Day After Operation | The 7th Day After Operation | On the 11th Postoperative Day | Variance Ratio | P-Value |
|---|---|---|---|---|---|---|---|---|
| Low molecular weight heparin group (n = 21) | 14.06 ± 4.81 | 36.40 ± 15.31 | 30.50 ± 13.55 | 98.94 ± 13.26 | 129.18 ± 85.17 | 83.94 ± 68.86 | 13.388 | 0.001 |
| Control group (n = 10) | 17.05 ± 7.84 | 20.65 ± 12.10 | 22.55 ± 8.86 | 22.18 ± 5.54 | 26.80 ± 7.65 | 24.17 ± 5.89 | 6.874 | 0.001 |
| t-value | 1.025 | 2.862 | 0.377 | 3.001 | 3.131 | 2.916 | - | - |
| P-value | 0.332 | 0.019 | 0.715 | 0.015 | 0.012 | 0.017 | - | - |
a Values are expressed as mean ± SD.
4.6. Comparison of Aspartate Aminotransferase Levels Between Two Groups Before and After Operation
There was no significant difference in the levels of AST between the two groups before the operation and on the third postoperative day (P > 0.05). However, there was a significant difference in the levels of AST between the two groups on the first, fifth, seventh, and eleventh postoperative days (P < 0.05). Additionally, there was a significant difference in the levels of AST between the two groups before and after the operation (P < 0.05) (Table 6).
| Groups | Preoperative | The First Day After Operation | On the Third Day After Operation | On the Fifth Day After Operation | The 7th Day After Operation | On the 11th Postoperative Day | Variance Ratio | P-Value |
|---|---|---|---|---|---|---|---|---|
| Low molecular weight heparin group (n = 21) | 17.98 ± 3.46 | 43.91 ± 14.77 | 28.60 ± 10.67 | 84.12 ± 67.28 | 84.67 ± 55.18 | 55.54 ± 38.96 | 23.793 | 0.001 |
| Control group (n = 10) | 19.38 ± 7.67 | 20.27 ± 11.58 | 20.41 ± 7.15 | 21.26 ± 3.51 | 23.61 ± 6.79 | 20.94 ± 8.65 | 7.994 | 0.001 |
| t-value | 0.871 | 4.115 | 1.219 | 3.065 | 3.292 | 2.764 | - | - |
| P-value | 0.406 | 0.003 | 0.254 | 0.013 | 0.009 | 0.022 | - | - |
a Values are expressed as mean ± SD.
5. Discussion
Anticoagulants are commonly used to prevent and treat various thromboembolic diseases. The mechanism of action of LMWH involves the binding of antithrombin and the irreversible inactivation of coagulation factor Xa, resulting in its anticoagulant effect with a half-life (t1/2) (11). This half-life is significantly longer than that of ordinary heparin, with LMWH’s t1/2 in vivo being about eight times that of ordinary heparin. Its bioavailability for anticoagulant factor Xa activity is three times that of ordinary heparin. After intravenous injection for 12 hours, the bioavailability of subcutaneous administration is nearly 100% (12). Once-daily dosing is sufficient and convenient. Compared to unfractionated heparin, LMWH is considered safer during the perioperative period of esophageal cancer surgery (13), leading to its increased popularity over the former.
Bleeding and thrombocytopenia are frequently observed side effects, while only a limited number of reports suggest that LMWH may cause liver function damage during the perioperative period of esophageal cancer surgery (14). Recent clinical trials investigating LMWH have focused on its safety profile, particularly regarding hepatotoxicity. These studies have shown that while LMWH is generally well-tolerated, there are instances of elevated liver enzymes in a small percentage of patients (15). For instance, trials involving patients with VTE and those undergoing surgery have monitored liver function, revealing that enzyme elevations often resolve upon discontinuation of therapy. The overall incidence of significant hepatotoxicity remains low, suggesting that LMWH can be safely administered in most populations, although careful monitoring is advised for those with pre-existing liver conditions (16).
According to clinical trials and prospective studies, the administration of LMWH during the perioperative phase of surgery for esophageal cancer was found to cause liver function impairment. The occurrence rate was estimated to be between 5% and 9%, with liver function damage being considered three times higher than the usual upper limit of ALT and AST (17). Studies have suggested that LMWH can induce mild oxidative stress in liver cells, potentially leading to transient liver enzyme elevations. The molecular mechanisms underlying this effect are still under investigation, with hypotheses focusing on the drug’s interaction with hepatic endothelial cells and its impact on blood flow and metabolism within the liver (18). Understanding these mechanisms is crucial for developing strategies to mitigate any adverse effects associated with LMWH therapy.
The increase in liver enzymes usually appears 5 - 8 days after heparin is used, and it returns to normal or improves within two weeks after stopping the drug. Similarly, the findings of this research indicated that 18 individuals diagnosed with esophageal carcinoma experienced hepatic impairment within 3 to 5 days following the administration of heparin. Moreover, there was an elevation in liver enzymes while bilirubin levels remained within the normal range (19). Liver protection drugs were used, and the liver function of 4 patients improved. The liver function of 14 patients did not improve significantly and returned to normal within one week after discontinuing low molecular weight heparin.
In their study, Premkumar et al. reported on a male participant who exhibited regular results in liver function testing (20). After esophageal cancer surgery, he began using low molecular weight heparin in the perioperative period, which resulted in transamination after six days. On the ninth day, transamination reached its highest point, and all other identified factors contributing to liver damage were excluded. Serological findings in recent studies have underscored the importance of monitoring liver function tests during LMWH treatment. Elevated serum levels of ALT and AST have been reported, particularly in patients with risk factors such as obesity, alcohol use, or concurrent medications affecting liver metabolism (21). These findings emphasize the need for routine screening of liver enzymes in patients receiving LMWH, especially in high-risk groups. Liver enzyme levels started to improve and return to their normal state within eight weeks after discontinuing the use of low molecular weight heparin.
During the perioperative period, the majority of individuals diagnosed with esophageal cancer do not exhibit any apparent symptoms (14). However, it is possible for them to experience symptoms like nausea, vomiting, and abdominal discomfort.
In this study, cholinesterase levels in patients with esophageal cancer decreased after the operation and generally began to rise gradually within five days, primarily manifesting as an asymptomatic increase in serum transaminase, which usually occurred in the first week of administration. Among the patients with hepatic insufficiency, more than half did not receive liver protection treatment, and about 10% discontinued anticoagulation treatment due to hepatic insufficiency. Nevertheless, the hepatic functionality of every individual gradually improved. For patients who did not receive LMWH, liver enzymes were generally normal after esophageal cancer surgery, with no obvious liver function damage.
A study by Bakshi et al. discovered that approximately 40% of individuals administered LMWH for 35 days following surgery for esophageal cancer experienced liver dysfunction. These patients exhibit no symptoms like nausea, vomiting, or jaundice. However, their liver test results began to increase from the first day of anticoagulant usage, reaching the highest point during the second week. Fortunately, liver function returned to normal without any intervention over time (22). Betancourt et al. also found that LMWH can cause liver function damage in patients with esophageal cancer during the perioperative period, which can return to normal after discontinuing the drug (23).
Regarding liver dysfunction caused by LMWH, it was discovered that seven patients experienced an increase in the levels of ALT, glutamyl transpeptidase, and AST. However, these patients did not show any symptoms (24). Following cessation of drug usage, liver function gradually normalized, with infrequent elevation of bilirubin levels and the majority of individuals displaying no clinical symptoms, aligning with the findings of this investigation. However, Saftoiu et al. observed that a patient diagnosed with esophageal carcinoma experienced nausea and vomiting after consuming LMWH for 48 hours. Additionally, there was an elevation in ALT, glutamyl transpeptidase, and AST levels. Upon modifying the medication, liver function returned to its usual state (25).
Furthermore, it should be emphasized that the use of LMWH, such as enoxaparin sodium, sodium heparin calcium, and fludarabine sodium, can potentially lead to mild liver impairment during clinical use. This typically manifests within the initial week of treatment but tends to resolve rapidly without any intervention or cessation of medication (26, 27). Clinical trials have demonstrated that the development of liver function impairment caused by LMWH encompasses mitochondrial function (28), immune response (29), signal transduction (30), drug metabolism (31), genetic factors (32), environmental influence (33), and various other factors. Senzolo et al. discovered that patients treated with LMWH or unfractionated heparin have a distinct hepatocyte biomarker called miR-122 in their serum. Furthermore, the liver is the sole origin of miR-122 found in circulation (34). Hence, it is hypothesized that enoxaparin sodium has the potential to directly impact the hepatocyte membrane, leading to the overall release of hepatocyte content (35, 36).
Moreover, healthcare professionals seldom consider the impact of LMWH on the liver while prescribing blood thinners, and its harmless, temporary, and reversible consequences may not justify thorough investigation. However, it must be known that higher doses and longer treatment times are related to a higher probability of liver injury, and the establishment of injury mechanisms and how to prevent it need further study (37, 38). Creating awareness about this issue can reduce unnecessary and excessive tests, patient distress, and healthcare expenses. In conclusion, the use of LMWH during the perioperative period of esophageal cancer can lead to liver function impairment. Clinically, we should pay close attention to changes in liver enzyme levels in patients. If liver function damage is evident, we should stop using LMWH and adopt other anticoagulation methods.