1. Background
Necroptosis is a novel alternative model of programmed cell death. Under certain conditions, the natural pathways of apoptosis may be inhibited, leading to necroptosis. Unlike apoptosis, necroptosis lacks key features such as nuclear fragmentation and excessive nuclear density; however, it shares characteristics with necrosis, such as the leakage of cellular contents outside the cell due to damage to the cell membrane (1). Necroptosis is a form of programmed cell death caused by the deregulation of specific molecules (1). The signaling processes involved in necroptosis depend on the kinases RIPK1, RIPK3, and MLKL, with MLKL serving as the final effector in the pathway. When activated, MLKL facilitates the formation of plasma membrane channels that cause membrane disruption (2).
Recent research indicates that necroptosis plays a significant role in various human diseases, including inflammatory, degenerative, and infectious conditions, particularly in liver injuries and diseases (3). In patients with alcoholic liver disease, the expression of RIPK3 in the liver is higher than in healthy individuals (4). Diseases linked to necroptosis may arise from exposure to specific conditions such as smoking, nanomaterials, and environmental heavy metal pollutants (5). Environmental heavy metals such as cadmium (Cd) are known to contribute significantly to the onset of necroptosis through molecular pathways that are still under investigation (6). For instance, Cd has been shown to increase the mRNA levels of RIPK1 and MLKL in cardiomyocytes, thereby inducing necroptosis (7). Cadmium is a prevalent environmental pollutant and is commonly introduced into the body through contaminated water and cigarette smoke, which are significant sources of exposure (8). The liver is one of the key organs exposed to Cd. Numerous studies have indicated that Cd can trigger autophagy, apoptosis, and necrosis in liver cells (9).
Exercise plays a crucial role in mitigating liver damage resulting from Cd exposure. In liver cells of rats exposed to Cd, it has been noted that endurance training can enhance apoptosis by elevating Bcl-2 levels (10). Swimming has also been found to have beneficial effects on indices of liver apoptosis induced by Cd (11). Furthermore, exercise acts to inhibit necroptosis. Leem et al. demonstrated that combining exercise with creatine supplementation reduced necroptosis activity by enhancing levels of MLKL, RIPK1, and RIPK3 (12). In another investigation, it was noted that exercise inhibited necroptosis in rats suffering from atrial fibrillation (AF) (13). However, the precise mechanism by which exercise enhances necroptosis signaling in the livers of Cd-exposed rats remains unclear.
In addition to physical activity, various natural substances and medicinal plants contribute to the improvement of liver function. Curcumin (Cu), the principal active compound in turmeric, is utilized as a natural food colorant and spice (14). Numerous studies have demonstrated that Cu protects against liver injury (15). In vivo experiments using a chemically induced liver fibrosis model revealed that Cu suppressed inflammatory factors in the liver. Furthermore, Cu helps to reduce liver fibrosis (16). The liver serves as a vital detoxification center and a key metabolic organ within the body, playing an essential role in defending against damage from external toxins. Unfortunately, research on the effects of exercise and Cu on the necroptosis pathways of liver tissue in the context of Cd poisoning is limited.
2. Objectives
The present study aimed to explore the potential mechanisms by which aerobic training (AT), combined with Cu consumption, counteracts Cd-induced necroptosis in the livers of rats. The researcher hypothesizes that the combination of AT and Cu consumption has a more beneficial effect on the necroptosis process in liver tissue. This study offers a new perspective on the mechanisms of targeted interventions through AT and Cu to diminish liver damage induced by Cd exposure.
3. Methods
Forty male Wistar rats (8 - 10 weeks, 190 - 220 g) were sourced from the laboratory animal breeding and reproduction center. To facilitate adaptation to their new environment, the rats were housed in the laboratory for one week. They were maintained under standard conditions, with unrestricted access to water and food. Following this acclimatization period, the rats were randomly allocated into five parallel groups: Control (C), Cd, Cd + Cu, Cd+ AT, and Cd+ Cu + AT. Eligibility criteria included being healthy, male, 8 - 10 weeks of age, and free from disease or infection prior to randomization. The present study received approval from the Ethics Committee at the Islamic Azad University, Ayatollah Amoli Branch (IR.IAU.AMOL.REC.1403.118).
3.1. Cadmium Supplementation
Pure Cd chloride was sourced from Sigma Aldrich. Based on the number and weight of the rats, 35 mg of Cd was dissolved in the daily water intake of the groups receiving Cd. The rats were administered 5 mg/kg of Cd dissolved in their drinking water daily (17).
3.2. Aerobic Training Protocol
The rats were familiarized with the treadmill for a week, running for 10 minutes each day at a speed of 8 m/min; 0% slope. Subsequently, they ran daily for 60 minutes at a speed of 15 m/min on a 15-degree slope for eight weeks, with five sessions each week. To adhere to the principle of overload, the exercise duration was set at 30 minutes in the first to fourth week, then increased to 60 minutes from the fourth to the eighth week (18).
3.3. Curcumin Supplementation
Initially, Cu was sourced from Sigma Aldrich in America. Subsequently, each mouse was administered a solution of 160 μL of Cu (dissolved in dextrose) per kilogram of body weight using small bottles (19).
3.4. Dissection and Sampling
Forty-eight hours after the end of the protocol, animals were anesthetized after a 12-hour fast. Once full anesthesia was achieved, the liver tissue was dissected, weighed, and rinsed. The tissue, after being frozen with liquid nitrogen, was stored in a refrigerator at -80°C. To mitigate the influence of circadian rhythms, the tissue collection commenced at 8:00 AM and concluded at 11:30 AM. The total RNA was extracted from the liver using RNA purification kits (Cinagene, Iran). Complementary DNA (cDNA) was measured based on the standard manufacturer’s protocol. The sequence of primers is listed in Table 1. Gene expression was examined using the quantitative q-RT PCR method.
| Genes | Primers (5’- 3’) | Product Lengths (bp) |
|---|---|---|
| MLKL | Forward 5′-CACCGATACCAGGAGCCAGT-3′ | 123 |
| Reverse 5′-AGCCAGCAAGTCCACGATCT-3′ | ||
| RIPK1 | Forward 5′-AAGGGCGTTTCATCCTGGAG-3 | 181 |
| Reverse 5′-CGGCAGGTCTCTTCTTTGGT-3′ | ||
| RIPK3 | Forward 5′-CCCATGGACAGGGAATGGAA-3′ | 112 |
| Reverse 5′-CCACAAGTCTCTGGTAGCGG-3′ | ||
| SIRT1 | Forward 5′-GACTCCAAGGCCACGGATAG-3′ | 136 |
| Reverse 5′-TGTTCGAGGATCTGTGCCAA-3′ | ||
| GAPDH | Forward 5′-GGTAGTGAAGGCTGCTGCTGATG-3′ | 200 |
| Reverse 5′-AGTCCACAACACGGTTGCTGTATC-3′ |
3.5. Data Analysis
One-way analysis of variance (ANOVA) and Tukey’s post hoc test were performed to evaluate the differences between the groups using SPSS software. A P-value of less than 0.05 was considered statistically significant.
4. Results
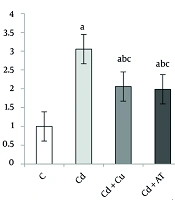
Data analysis showed a significant difference in the level of MLKL changes in the liver between different groups (P = 0.0001, F = 13.415). Tukey’s test indicated an increase in MLKL levels in the Cd (P = 0.0001), Cd + Cu (P = 0.026), and Cd + AT (P = 0.044) groups compared to the C group. A significant decrease was observed in the Cd + Cu (P = 0.040), Cd + AT (P = 0.024), and Cd + Cu + AT (P = 0.0001) groups compared to the Cd group; and in the Cd + Cu + AT group compared to the Cd + Cu (P = 0.018) and Cd + AT (P = 0.032) groups (Figure 1).
Liver expression of MLKL by one-way analysis of variance (ANOVA) (P < 0.05). a, difference from the C group; b, difference from the Cd group; and c, difference from the Cd + Cu + AT group. Abbreviations: C, control; Cd, cadmium; Cd + Cu, cadmium + curcumin; Cd + AT, cadmium + aerobic training; Cd + Cu + AT, cadmium + curcumin + aerobic training.
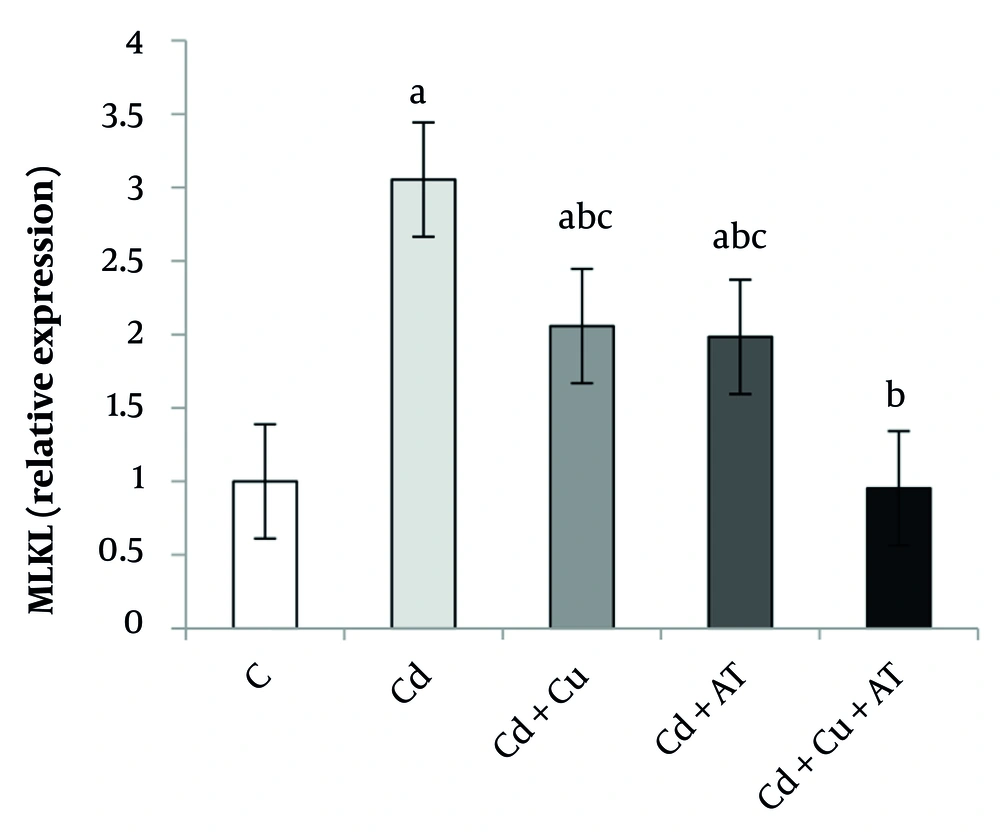
Data analysis also revealed a significant difference in RIPK1 expression in the liver between different groups (P = 0.0001, F = 13.589). Tukey’s test results showed a significant increase in RIPK1 in the Cd (P = 0.0001), Cd + Cu (P = 0.011), and Cd + AT (P = 0.012) groups compared to the C group. A significant decrease was observed in the Cd + Cu (P = 0.048), Cd + AT (P = 0.045), and Cd + Cu + AT (P = 0.0001) groups compared to the Cd group; and in the Cd + Cu + AT group compared to the Cd + Cu (P = 0.033) and Cd + AT (P = 0.035) groups (Figure 2).
Liver expression of PIPK1 by one-way analysis of variance (ANOVA) (P < 0.05). a, difference from the C group; b, difference from the Cd group; and c, difference from the Cd + Cu + AT group. Abbreviations: C, control; Cd, cadmium; Cd + Cu, cadmium + curcumin; Cd + AT, cadmium + aerobic training; Cd + Cu + AT, cadmium + curcumin + aerobic training.
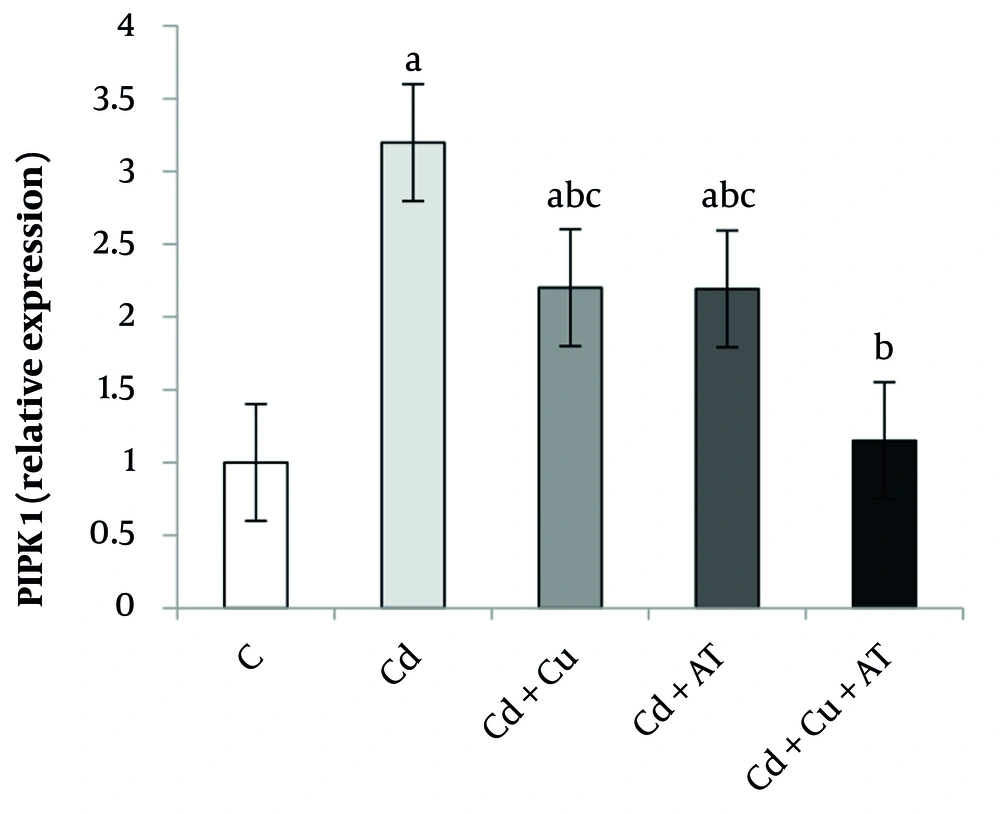
Among other results, there was a significant difference in the expression level of RIPK3 in the liver between different groups (P = 0.0001, F = 10.549) (Figure 3). Tukey’s test showed a significant increase in RIPK3 expression in the Cd group (P = 0.0001) compared to the C group. A significant decrease was observed in the Cd + Cu (P = 0.047), Cd + AT (P = 0.042), and Cd + Cu + AT (P = 0.0001) groups compared to the Cd group (Figure 3).
Liver expression of PIPK3 by one-way analysis of variance (ANOVA) (P < 0.05). a, difference from the C group; b, difference from the Cd group; and c, difference from the Cd + Cu + AT group. Abbreviations: C, control; Cd, cadmium; Cd + Cu, cadmium + curcumin; Cd + AT, cadmium + aerobic training; Cd + Cu + AT, cadmium + curcumin + aerobic training.
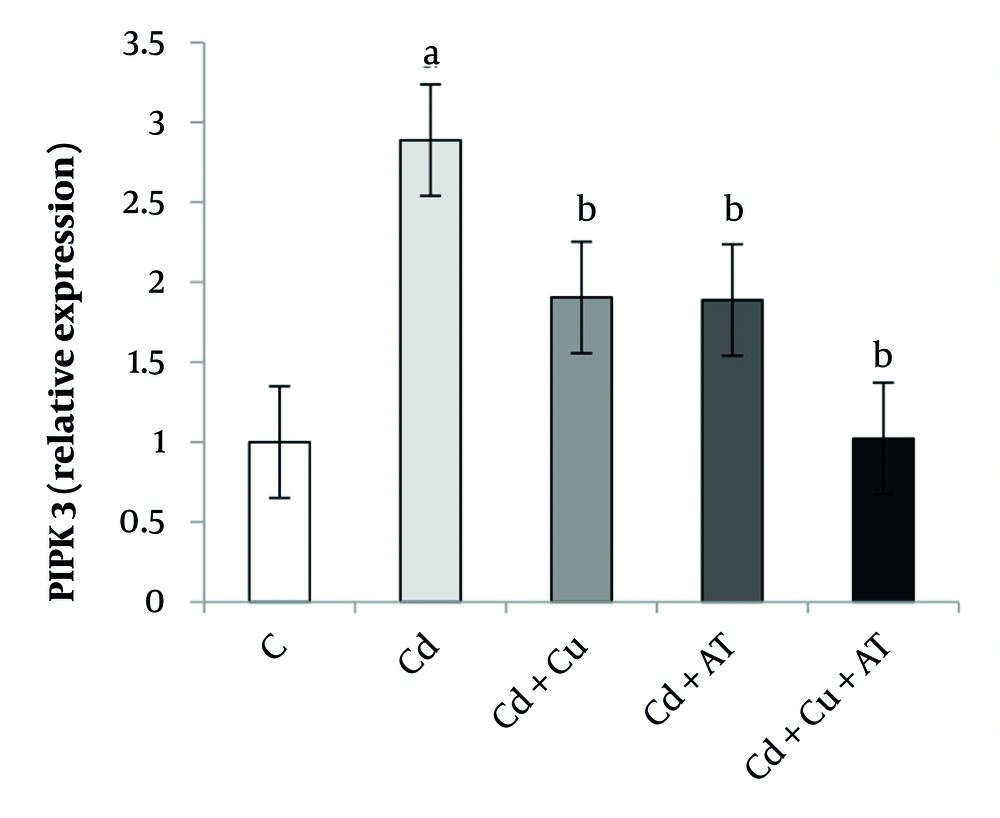
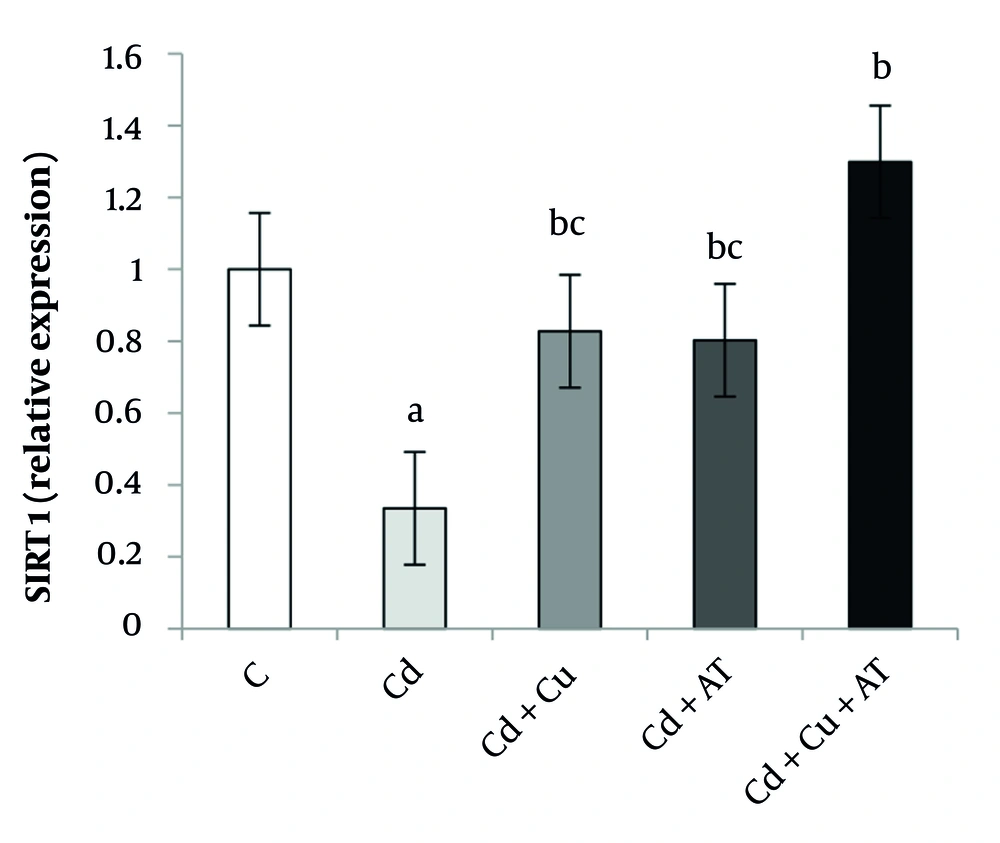
Finally, data analysis showed a significant difference in the expression level of SIRT1 in the liver between different groups (P = 0.0001, F = 9.927). Tukey’s test results indicated a significant decrease in SIRT1 levels in the Cd group (P = 0.001) compared to the C group. A significant increase was observed in the Cd + Cu (P = 0.027) and Cd + AT (P = 0.040) groups compared to the Cd group; and in the Cd + Cu + AT group compared to the Cd + Cu (P = 0.038) and Cd + AT (P = 0.026) groups (Figure 4).
Liver expression of SIRT1 by one-way analysis of variance (ANOVA) (P < 0.05). a, difference from the C group; b, difference from the Cd group; and c, difference from the Cd + Cu + AT group. Abbreviations: C, control; Cd, cadmium; Cd + Cu, cadmium + curcumin; Cd + AT, cadmium + aerobic training; Cd + Cu + AT, cadmium + curcumin + aerobic training.
5. Discussion
The present study aimed to investigate the effects of AT and Cu on necroptosis markers in Cd-exposed rat liver tissue. The main findings revealed that Cd exposure significantly increased the expression of MLKL, RIPK1, and RIPK3, while decreasing SIRT1 expression. Both AT and Cu intervention individually reversed these changes, and their combination had a more significant effect. Cadmium hepatotoxicity is primarily manifested as liver dysfunction, inflammation, necrosis, fat accumulation, and fibrosis. Previous research indicates that Cd-induced hepatotoxicity involves apoptosis, calcium homeostasis disruption, oxidative stress, and inflammation (20).
The findings of the present study revealed that Cd poisoning was linked to an increased expression of MLKL, RIPK1, and RIPK3, alongside a decrease in SIRT1 in the liver tissue of mice. In this context, Liu et al. demonstrated that exposure to Cd significantly elevated Cd accumulation and liver function markers (ALT and AST), correlating with increased necroptosis as well as apoptosis in the liver (21). Additionally, Zhang et al. reported that Cd induces necroptosis and other forms of liver damage both in vivo and in vitro (22). Xia et al. suggested that exposure to Cd likely triggers necroptosis through the activation of the STAT1/RIPK3 signaling pathway due to elevated ROS levels. Another mechanism of necroptosis activation involves the disruption of the endoplasmic reticulum (23). However, our study did not evaluate upstream regulatory mechanisms of necroptosis, such as TNF-α levels, mitochondrial dysfunction, and oxidative stress, which represent limitations and potential areas for future research (24).
Study by Cui et al. observed that Cd induces endoplasmic reticulum stress by disturbing the Th1/Th2 balance, subsequently leading to necroptosis via the activation of the RIPK1/RIPK3/MLKL signaling pathway (25). Nevertheless, in the current study, AT was found to reverse the effects of Cd on necroptosis and to enhance the expression of SIRT1. Consistent with this study, Leem et al. demonstrated that exercise combined with creatine supplementation improved necroptosis activity in Parkinson’s rats by reducing the expression of MLKL, RIPK1, and RIPK3, while also enhancing inflammatory and antioxidant status (12). Similarly, Fu et al. observed that swimming training for 60 minutes a day over three months in rats induced with AF was associated with a decrease in the expression of RIPK1, RIPK3, and MLKL (13).
Although SIRT1 expression was increased by exercise and Cu in our study, the causal relationship between SIRT1 upregulation and necroptosis inhibition remains speculative due to the lack of direct mechanistic evidence (26). Exercise has the ability to activate the PGC-1α-SIRT1 signaling pathway (27), thereby potentially affecting the necroptosis process. Furthermore, a correlation was observed between TNFα-induced necroptosis and oxidative stress. Oxidative stress-induced necroptosis is correlated with the activation of downstream signaling pathways of RIP3, such as CaMKII (28). On the other hand, RIPK activity results in the generation of reactive oxygen species (ROS) (29). As previously mentioned, endoplasmic reticulum stress also plays a significant role in the onset of necroptosis. Studies by Kazemi et al. have demonstrated that physical activity can inhibit endoplasmic reticulum stress in the liver of NAFLD rats (30).
Cadmium exposure is linked to increased Drp1 expression, mitochondrial dysfunction, and hepatic necroptosis. The up-regulation of Drp1 expression through binding to RIPK3 within the mitochondrial compartment leads to liver necroptosis (22). In this context, it has been shown that AT can diminish Drp1 expression (31). It appears that in the current study, physical activity has mitigated necroptosis by inhibiting oxidative stress, lowering Drp1 expression and endoplasmic reticulum stress, as well as improving both inflammatory and oxidative states. Nevertheless, in the research conducted by Zeini Zadeh et al. involving rats with Alzheimer’s disease, it was noted that exercising on a rotating wheel, despite its positive effects on the disease, did not significantly impact indicators of necroptosis (32). The differences in findings are likely attributable to the type of disease, the tissue studied, and the intensity of the exercise.
The results of the current study demonstrated that Cu consumption was effective in reducing the expression of MLKL, RIPK1, and RIPK3 in the liver tissue of rats, while it increased the expression of SIRT1. The reduction of necroptosis following Cu intake was reported in the research conducted by Li et al. The authors of this study indicated that Cu can lower inflammatory cytokine and oxidative stress, diminish the expression of necroptosis and regulate the TLR4/RIPK signaling pathway (33). Furthermore, the findings from Sun et al. revealed that Cu inhibits necroptosis by mitigating endoplasmic reticulum stress and the SIRT1 signaling pathway (34). Necroptosis is frequently linked to increased inflammation, ROS, and oxidative stress, and Cu is capable of reducing MDA and ROS levels while enhancing SOD and CAT in liver tissue, thereby providing protection against necroptosis (33). Additionally, Cu plays a crucial role in controlling necroptosis by regulating inflammatory markers (33).
According to the findings of this study, the consumption of Cu may decrease the expression of RIPK1/RIPK3 by inhibiting TLR4 and TNF-α signaling, and it may also hinder Cd-induced liver necroptosis in mice. Curcumin is capable of reducing endoplasmic reticulum stress and the necrosome markers RIP1 and RIP3 in liver cells, making it an effective treatment strategy for liver fibrosis (34). Furthermore, Cu can mitigate endoplasmic reticulum stress and necroptosis through the regulation of the SIRT1/Notch pathway (34).
The combination of exercise and Cu has a more significant effect on liver necroptosis in rats exposed to Cd than either intervention alone. Our research found that the simultaneous impact of exercise and Cu on hepatic necroptosis has not been previously examined. It is important to note that the study was conducted only on male rats, and sex-based differences were not evaluated. Prior research has demonstrated sex differences in hepatic oxidative response to toxins, which may influence necroptotic pathways. In addition, this limitation may reduce the generalizability of the findings (35).
However, earlier studies have demonstrated that AT combined with Cu can effectively reduce factors influencing apoptosis (36) and balance oxidative stress. Furthermore, Sadeghian et al. investigated the effects of AT alongside Cu on the gene expression of apoptosis markers in the liver tissue of cancerous rats undergoing treatment with doxorubicin. Their findings revealed that the combination of exercise and Cu supplementation was linked to an increase in Bcl-2 expression and a decrease in caspase-3 levels (37). Additionally, Majidi et al. observed that combining high-intensity interval training with Cu reduced the expression of caspase-3 and miR-1, while elevating miR-133 expression in rats exposed to arsenic (38).
It appears that the synergistic effects of exercise and Cu have yielded these results. This synergy can be attributed to the effectiveness of both AT and Cu on pathways that influence necroptosis, including the SIRT1 signaling pathway, endoplasmic reticulum stress, oxidative stress, and inflammatory factors. One of the limitations of the present study is the absence of measurement of upstream factors such as TNF-α, ROS, and endoplasmic reticulum stress levels, which could provide a more integrated understanding of necroptosis regulation. Furthermore, the analysis did not account for potential imprecision in biomarker quantification or biological variability. These limitations should be addressed in future studies to enhance the robustness of findings.
The findings of this study indicate that exposure to Cd correlates with an increase in markers of necroptosis. Engaging in AT and taking Cu supplements may lead to a reduction in the expression of MLKL, RIPK1, and RIPK3, alongside an increase in SIRT1 levels. Furthermore, the combined effect of AT and Cu on these markers was found to be more beneficial than each intervention on its own. It appears that both exercise and Cu can partially mitigate Cd-induced liver damage by inhibiting necroptosis.