1. Background
Liver fibrosis is a progressive disorder characterized by the excessive accumulation of extracellular matrix (ECM) components, leading to scarring and impaired liver function (1, 2). This condition often arises from chronic liver injury, which triggers an inflammatory response and activates hepatic stellate cells (HSCs) to transform into myofibroblasts (MFBs) (3, 4). While this transformation is crucial for the repair process, excessive activation can lead to overproduction of ECM, disrupting the normal architecture of the liver and potentially advancing to cirrhosis and hepatocellular carcinoma (HCC) (5). Understanding the mechanisms underlying liver fibrosis is essential for developing effective therapeutic strategies. The accumulation and crystallization of cholesterol in hepatic cells among patients with nonalcoholic steatohepatitis (NASH) can result in cell death and subsequent liver fibrosis, a condition that is a leading cause of mortality worldwide (6, 7). Liver fibrosis initially arises as an immune response to chronic injury triggered by various factors, including metabolic and genetic liver disorders, viral infections, toxic substances, autoimmune hepatitis, and dietary influences. All these factors can contribute to the progression of cirrhosis and HCC (8). This condition is marked by the activation of HSCs into MFBs, the synthesis and deposition of ECM, and progressive inflammation (9). The regulation of ECM balance involves two key enzymes: Matrix metalloproteinases (MMPs), which break down the matrix created during tissue repair, and tissue inhibitors of metalloproteinases (TIMPs), which inhibit MMP activity. An imbalance between the degradation and accumulation of ECM components ultimately leads to fibrosis (10). The HSC activation is influenced by different inflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α). Transforming growth factor-beta (TGF-β), a key profibrotic cytokine in the liver, promotes the expression of collagen I and alpha-smooth muscle actin (α-SMA) through the activation of the SMAD2/3 pathway (11, 12). The activation of NADPH oxidase (NOX) enzymes and the subsequent overproduction of reactive oxygen species (ROS) play a critical role in activating HSCs and increasing ECM synthesis associated with liver fibrosis. NADPH oxidase is an enzyme complex located in the cell membrane that creates ROS when triggered by various external factors, making it the fundamental source of ROS in the liver. Recent studies have shown that factors such as TGF-β (13, 14), angiotensin II, and platelet-derived growth factor (PDGF) promote profibrotic differentiation in HSCs via the activation of phagocytic NOX2 as well as nonphagocytic isoforms like NOX1 and NOX4. This signaling pathway underscores the relevance of oxidative stress in the fibrotic process and highlights potential therapeutic targets for managing hepatic fibrosis (15).
Increased intake of dietary cholesterol activates HSCs and increases their sensitivity to TGF-β-induced activity as a result of the buildup of free cholesterol in these cells. The harmful effects of lipids, particularly cholesterol, play a significant role in the advancement of liver fibrosis and cirrhosis (16). Wharton jelly mesenchymal stem cells (WJ-MSCs) have the exceptional capacity to differentiate into multiple cell types at various stages of maturation. They are ethically sourced, easily obtainable, and considered safe for therapeutic use (17). However, there are some limitations linked to the use of WJ-MSCs, including the risk of cell rejection, suboptimal grafting outcomes, and a limited lifespan following transplantation into target tissues (18, 19). Research has demonstrated that mesenchymal stem cells (MSCs) can provide therapeutic benefits through their paracrine functions. The therapeutic potential of MSCs is primarily attributed to extracellular vesicles, including microvesicles and exosomes, which facilitate intercellular communication, transfer paracrine factors, and promote tissue regeneration (20, 21). Exosomes are tiny vesicles, measuring approximately 30 to 100 nm in diameter, that are produced and released by numerous cell types, including MSCs. They transport proteins and nucleic acids, such as messenger RNA (mRNA) and microRNAs (miRs), which are crucial for regulating cellular signaling pathways (22, 23). Polyinosinic:polycytidylic acid (poly I:C) exhibits significant preventive and antifibrotic effects on liver fibrosis, which are associated with elevated concentrations of interferon-gamma (IFN-γ). The poly I:C therapy not only induces apoptosis and cell cycle arrest in HSCs but also enhances the cytotoxic activity of natural killer (NK) cells against active HSCs (24, 25). However, the mechanisms underlying the anti-inflammatory properties and actions of poly I:C remain largely unknown.
2. Objectives
The present study focused on examining the impacts of mesenchymal stem cell-derived exosomes activated with poly I:C on the regulation of collagen I and α-SMA genes triggered by the buildup of free cholesterol in the LX2 cell line, as well as the phosphorylation of the SMAD3 signaling pathway.
3. Methods
3.1. Hepatic Stellate Cells Cultivation and Treatment
LX2 cells (human HSCs) were cultured following the provider’s protocol. The cells were maintained in a 6-well plate containing Dulbecco’s Modified Eagle Medium (DMEM) enriched with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 environment and were passaged when they attained 80% to 90% confluence. To activate LX2 cells and establish a liver fibrosis model, they were starved for 16 hours, then treated with 100 μg/mL cholesterol (Sigma-Aldrich, USA) (26). The therapeutic effect of exosomes was assessed by adding 50 μg/mL exosomes (17) to the cells in an FBS-free culture medium for 24 hours. To evaluate the effect of poly I:C (Sigma-Aldrich, USA) stimulated exosomes at a dosage of 10 μg/mL (27), they were prepared in an FBS-free culture medium and introduced to LX2 cells.
3.2. Extraction and Cultivation of Wharton Jelly Mesenchymal Stem Cells
Following the acquisition of informed consent, a fresh human umbilical cord (UC) was collected post-cesarean section, washed with phosphate-buffered saline (PBS) containing penicillin and streptomycin, and transported to the lab at 4°C. Procedures were conducted in a class 2 laminar flow hood. The Wharton’s jelly was cut into 1- to 2-mm fragments, washed, and placed into 25-cm flasks for 5 minutes to promote adhesion. To prevent floating, the upper parts of the tissue pieces remained above the culture medium, which included low-glucose DMEM enriched with 20% FBS and antibiotics. Cells were harvested when they reached 80% to 90% confluence and transferred to new plates at a density of 8 × 103 cells/cm2, with passages 3 to 5 exclusively used for the experiment.
3.3. Evaluation of Wharton’s Jelly Mesenchymal Stem Cell Differentiation
This research focused on WJ-MSCs from passages 3 to 5, which were subjected to osteogenic and adipogenic differentiation assays. For osteogenic differentiation, cells were plated at 20,000 cells/mL in six-well plates and maintained in osteogenic medium with 10% FBS, 10 mM β-glycerophosphate, 10 nM dexamethasone, and 50 µg/mL ascorbic acid. Media was refreshed twice per week over a 21-day period. Afterward, the cells were preserved in 10% formaldehyde solution for 10 minutes and subjected to staining with 1% Alizarin Red S (Sigma-Aldrich, USA) for 2 minutes, followed by analysis using a confocal microscope. For adipogenic differentiation, cells were grown in α-MEM containing 10% FBS, along with indomethacin, dexamethasone, and ascorbic acid for 21 days. After fixation, they were stained with 0.5% Oil Red O and examined under a confocal microscope to visualize lipid droplets.
3.4. Evaluation of WJ-MSC Surface Proteins Using Flow Cytometry
After preparing a 1 × 106 hWJMSC solution, the cells were stained with antibodies labeled with various fluorescent probes. The antibodies obtained from a bioscience company included PE-labeled mouse monoclonal antibody against human CD44 and CD105, and FITC-labeled mouse anti-human CD34 and CD45. The cells were incubated for 30 minutes before being washed and suspended for examination using a BD FACS Lyric instrument (Dickinson, USA).
3.5. Induction of Wharton’s Jelly Mesenchymal Stem Cells with Polyinosinic: Polycytidylic Acid
In this study, the culture medium was replaced once the cell density reached 80%. Subsequently, the cells were cultivated with a concentration of 10 μg/mL poly I:C (27) adjuvant, which was dissolved in the culture medium, for a duration of 72 hours.
3.6. Extraction of Exosomes
To extract exosomes from WJ-MSCs upon achieving 90% confluence, the FBS concentration was gradually reduced by approximately 2% every three days until the culture medium contained no FBS. Following a 72-hour incubation period in the serum-free medium, the cell culture supernatant was collected and filtered through a 0.22-micrometer filter to eliminate cell debris. Exosomes were subsequently isolated using the EXOCIB isolation kit (CIB Biotech, Iran). The resulting exosomes were resuspended in PBS and stored at -80°C for future use.
3.7. Exosome Characterization
The structure of exosomes was analyzed through transmission electron microscopy (TEM). Exosomes were fixed in 1% glutaraldehyde for 20 minutes, placed on carbon-coated grids, and air-dried. After washing with sterile PBS and staining with 1% uranyl acetate, their morphology and size were analyzed with a TEM LEO 906 microscope. Dynamic light scattering (DLS) was also employed to assess exosome dimensions and dispersal, with samples adjusted to a concentration of 1 µg/mL in PBS containing 0.05% Tween-20, and analyzed at 23°C using a Zetasizer Nano.
3.8. RNA Extraction and Quantitative RT-PCR Analysis
Total RNA was extracted using the RNA Isolation Kit (Yekta Tajhiz Azma, Iran), and complementary DNA (cDNA) synthesis followed using a kit from the same manufacturer. Real-time PCR was conducted with SYBR Green master mix (Ampliqon, Denmark) on the QuantStudio™ 3 real-time PCR System (ABI Applied Biosystems), following the provided protocols. Primers were designed by Sinaclon (Tehran, Iran) and are listed in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the reference gene for normalization, and the 2-ΔΔCT method was used to calculate relative gene expression changes.
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| COL1A1 | GTGGACATCAACGGGTTCACT | CTCCGTGGAGCTGAAGCAATA |
| α-SMA | TCGGATACTTCAGCGTCA | GGGAGTAATGGTTGGAATG |
| TGF-β | GGGAGTAATGGTTGGAATG | GGGAGTAATGGTTGGAATG |
| NOX1 | CTGTTGCCTAGAAGGGCTCC | ACAGGCCAATGTTGACCCAA |
| NOX2 | GTTGCCCGAGATGCCAATTC | CATGTCCAGGAATCGCTCCA |
| NOX4 | TGGAGGAAGAGGGAAGAGGT | AGAGCCAGATGAACCCAAGC |
| GAPDH | GACAGTCAGCC GCATCTTCT | GCCCAATACGACCAAATCCGT |
The Primer Sequences of Gene
3.9. Protein Extraction and Analysis via Western Blot
The levels of phosphorylated Smad3 (p-Smad3) protein were assessed through Western blot analysis. The HSCs were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors. Protein concentrations were measured with a bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific, USA). Each protein sample (30 µg) underwent separation via SDS-PAGE, followed by transfer to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). Membranes were treated overnight at 4°C with a primary antibody (1:1000; Cell Signaling, USA), rinsed three times with Tris-buffered saline with Tween 20 (TBST), and then incubated with a secondary antibody (1:10,000; Cell Signaling, USA). Bound antibodies were detected with an enhanced chemiluminescence (ECL) kit (GE Healthcare, IL, USA), and protein bands were visualized using a Bio-Rad ChemiDoc imaging system. The GAPDH was used as the internal control for normalization, and band intensities were analyzed using NIH ImageJ software.
3.10. Assessment of Reactive Oxygen Species Production
In this assay, reactive species are detected using dichlorodihydrofluorescein diacetate (DCFH-DA). This compound penetrates living cells, where it is de-esterified by intracellular esterases and subsequently retained within the cell. Upon being reduced by ROS, it displays fluorescence properties. Fluorescence intensity was recorded with a fluorometer set to an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
3.11. Analysis of Statistical Data
Data were analyzed using SPSS software version 24 (IBM Corporation, USA). Statistical significance among the groups was assessed employing analysis of variance (ANOVA) alongside Tukey’s multiple comparison tests, utilizing GraphPad Prism 9 software. The results derived from the experiments, conducted in triplicate, were thoroughly evaluated, and a P-value of less than 0.05 (P < 0.05) was considered statistically significant.
4. Results
4.1. Characterizing Wharton’s Jelly Mesenchymal Stem Cells
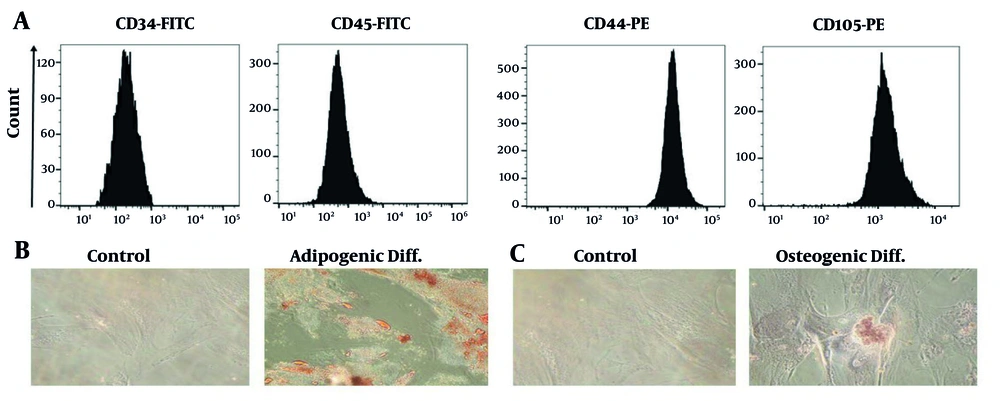
The surface markers of MSCs were analyzed using flow cytometry, showing that the purified cells lacked hematopoietic stem cell and monocyte macrophage markers but were positive for MSC markers such as CD44 and CD105 (Figure 1A). These antibodies did not differentiate mesenchymal cells from epithelial cells, blood cells, or myeloid lineages. In adipogenic differentiation medium, MSCs stained using Oil Red O revealed red intracellular lipid vacuoles, indicating adipocyte differentiation (Figure 1B). Additionally, cells grown in osteogenic differentiation medium and stained using Alizarin Red confirmed the calcification of the ECM, indicating osteogenic differentiation (Figure 1C).
Immunophenotypic analysis was conducted using flow cytometry to evaluate the surface markers of Wharton’s jelly mesenchymal stem cells (WJ-MSCs). A, The results indicated that WJ-MSCs express CD44 and CD105, while the expression of CD34 and CD45 was significantly down-regulated; B, On day 21, staining with oil red O showed bright red intracellular lipid accumulation, confirming adipogenic differentiation; C, Alizarin red S staining on day 21 revealed bright orange-red calcium deposition in osteocytes, indicating successful osteogenic differentiation.
4.2. Characterization of Exosomes
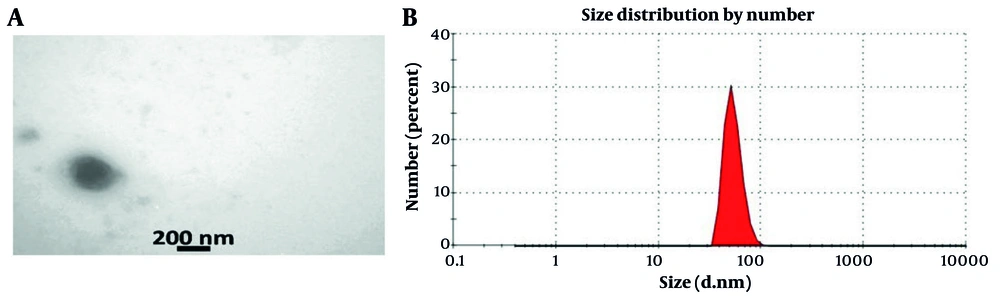
The analysis indicated that the isolated exosomes were spherical, with diameters between 50 and 200 nm (Figure 2A). Using a Malvern Zetasizer, the size distribution of these exosomes was assessed, showing that approximately 85% had a size of 73 nm in diameter (Figure 2B).
The characterization of exosomes involved several techniques. Transmission electron microscopy (TEM) was utilized to visualize the shape and morphology of the exosomes. Additionally, the Malvern Zeta Sizer was employed to determine their size distribution. The analysis revealed that up to 85% of the total exosomes measured approximately 73 nanometers in diameter, indicating a consistent size profile.
4.3. Impact of Cholesterol and Exosomes on Liver Fibrosis-Associated Gene Expression
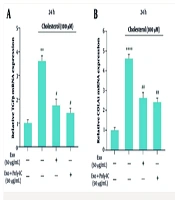
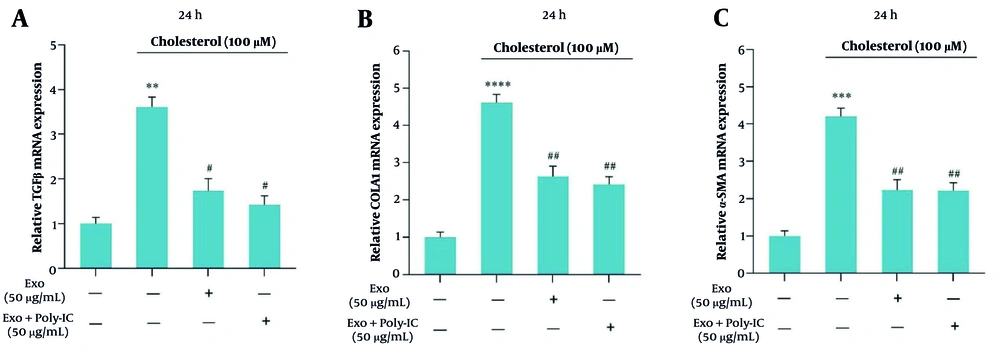
The quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis demonstrated a significant increase in the mRNA levels of TGF-β, α-SMA, and collagen1 genes in response to cholesterol treatment, indicating that cholesterol promotes the development of fibrosis in LX2 cells. Specifically, TGF-β expression, illustrated in Figure 3A, showed a marked elevation, while collagen1 expression, represented in Figure 3B, and α-SMA expression, depicted in Figure 3C, also increased significantly under cholesterol influence. Notably, treatment with unstimulated exosomes at a concentration of 50 µg/mL effectively reduced the expression levels of these genes. Furthermore, the application of exosomes stimulated with poly I:C resulted in an additional decrease in the expression of TGF-β, collagen1, and α-SMA genes, underscoring the potential of these exosomes in mitigating cholesterol-induced fibrotic changes.
The figure illustrates the impact of cholesterol and exosomes on the modulation of gene expression associated with the progression of liver fibrosis. Specifically, it presents the results from three independent replicates (Mean ± SEM) assessing the expression levels of TGF-β, collagen I, and α-SMA genes in the LX2 cell line treated with cholesterol and exosomes. The findings indicate a statistically significant difference with a significance level below 0.05. The notation used for significance is as follows: ** P < 0.01, *** P < 0.001, **** P < 0.0001, with the reference gene being noted as # P < 0.05, ## P < 0.01.
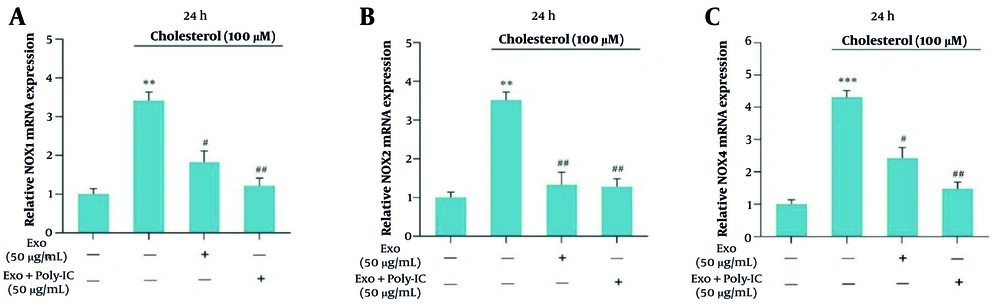
4.4. Exosomes’ Influence on NADPH Oxidase Gene Expression
Exosomes were analyzed for their effects on the mRNA expression levels of NOX1, NOX2, and NOX4 genes at a cholesterol concentration of 100 μM after 24 hours of incubation. The findings showed that unstimulated exosomes led to a significant reduction in the expression of these NOX genes. Furthermore, poly I:C-stimulated exosomes caused an even more pronounced decrease in NOX gene expression compared to unstimulated exosomes, with the most notable reductions observed in NOX1 and NOX4 (Figure 4A - C). Specifically, Figure 4A illustrates the impact on NOX1, Figure 4B depicts the effects on NOX2, and Figure 4C shows the alterations in NOX4 expression in fibrotic cells.
The effect of exosomes on the expression of NOX genes was examined in LX2 cells treated with cholesterol (100 μM) for 24 hours, with both unstimulated and poly I:C-stimulated WJ-MSCs used for comparison. A significance level of P < 0.05 was established, with the following notations: ** P < 0.01, *** P < 0.001, # P < 0.05, ## P < 0.01. The results presented are the means ± SEM from three independent replicates of the control group.
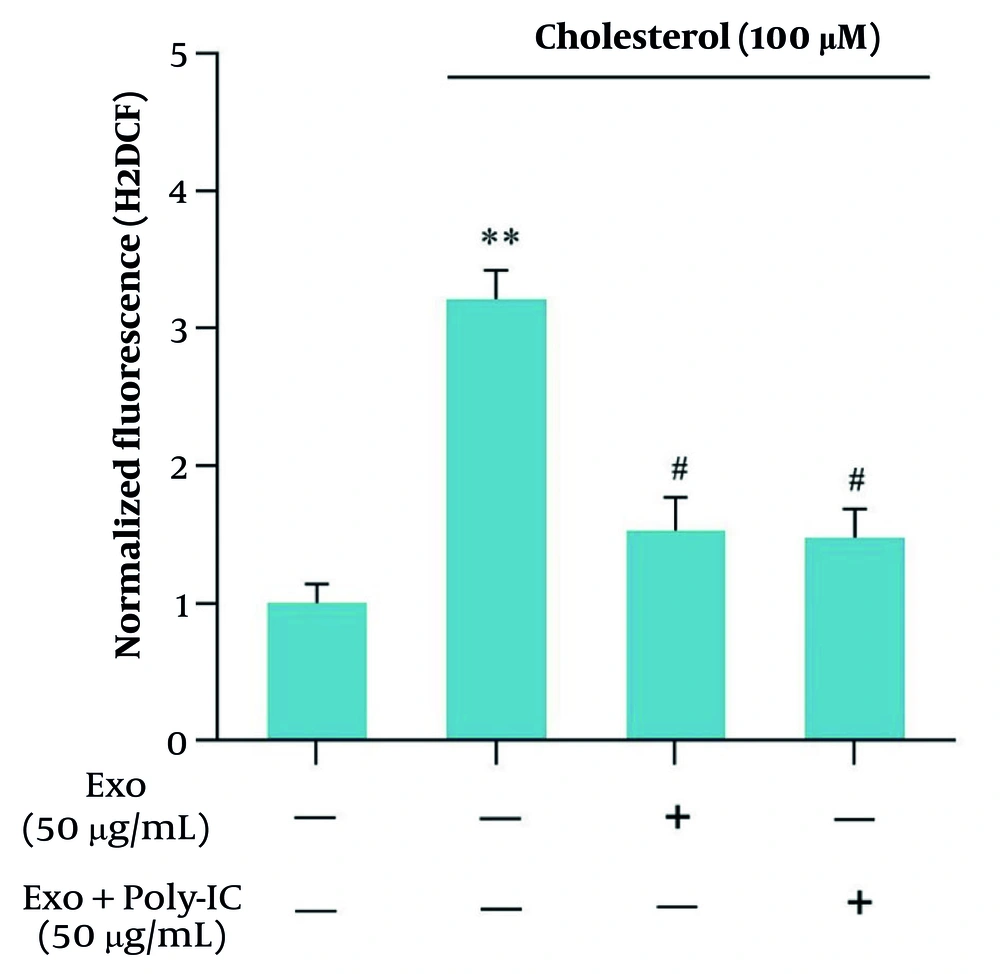
4.5. Impact of Cholesterol and Exosomes on Oxidative Imbalance in Hepatic Stellate Cells
The generation of ROS in LX2 cells treated with 100 μM cholesterol was evaluated as an indicator of oxidative stress associated with liver fibrosis progression. Subsequently, we assessed the impact of unstimulated WJ-MSC-derived exosomes on ROS production. The results demonstrated a notable increase in levels of ROS in the cholesterol-treated group relative to the control. Conversely, both unstimulated and poly I:C-stimulated exosome groups exhibited a significant reduction in ROS production (Figure 5).
The presence of cholesterol led to an increase in reactive oxygen species (ROS) levels, while treatment with exosomes exhibited a reducing effect on ROS production. Notably, there was no significant difference between polyinosinic: Polycytidylic acid (poly I:C)-stimulated exosomes and non-stimulated exosomes regarding ROS levels. The results presented are the means ± SEM from three independent replicates of the control group, with a significance level established at P < 0.05 (** P < 0.01, # P < 0.05).
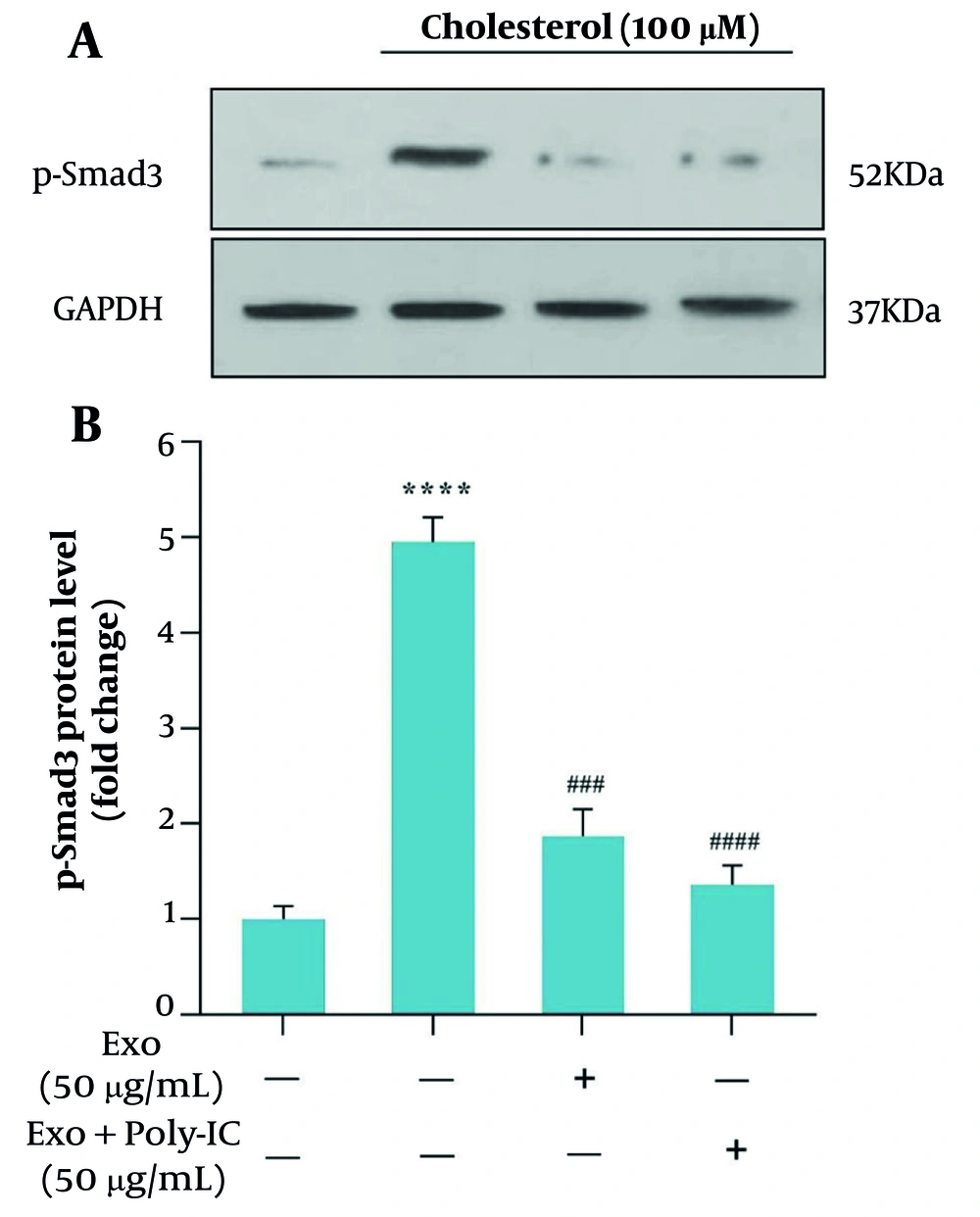
4.6. Effects of Cholesterol and Exosome Treatment on Smad3 Expression Level
We examined how exosomes influence the TGF-β/Smad3 signaling pathway in HSCs after cholesterol stimulation. After a 24-hour treatment with cholesterol at a concentration of 100 μM, cells were incubated for 1 hour with exosomes (50 μg/mL). Western blot analysis showed a marked rise in p-Smad3 levels after cholesterol treatment compared to the control group (Figure 6A). Importantly, treatment with WJ-MSC-derived exosomes markedly reduced the cholesterol-induced elevation of p-Smad3 (Figure 6B). Additionally, co-treatment with poly I:C and exosomes (50 μg/mL) further downregulated p-Smad3, resulting in decreased levels compared to the exosome-only treated group.
The effect of mesenchymal stem cell-derived exosomes (MSCs-Ex), whether stimulated with poly (I:C) or unstimulated, on cholesterol-induced activation of hepatic stellate cells (HSCs) was assessed by measuring p-Smad3c protein levels through Western blot analysis. A significance level of P < 0.05 was established, with GAPDH serving as an internal control gene for normalization (**** P < 0.0001, ### P < 0.001, #### P < 0.0001).
5. Discussion
Cholesterol accumulation in liver cells of individuals with NASH leads to cell death and fibrosis, significantly increasing mortality rates. This fibrotic process is marked by the activation of HSCs (28, 29), synthesis of ECM, and chronic inflammation, driven by an imbalance in ECM turnover influenced by cytokines, growth factors, and ROS (30). The TGF-β is particularly important, promoting collagen I (Col-alpha1) and α-SMA expression through SMAD2/3 signaling pathways. NADPH oxidase enzymes, especially NOX1 and NOX4, play an essential role in HSC activation and ECM synthesis, exacerbated by high cholesterol intake, which heightens sensitivity to TGF-β and contributes to the progression of NASH, fibrosis, and cirrhosis (31, 32). The mechanism of liver fibrosis involves HSC activation and trans differentiation into MFBs, leading to excessive ECM deposition. Chronic inflammation and oxidative stress, mainly through ROS, enhance TGF-β signaling, amplifying the fibrotic response. SMAD3, a key mediator of TGF-β, drives collagen and α-SMA expression, promoting fibrosis. High cholesterol exacerbates this process by increasing ROS production, which further activates HSCs and amplifies inflammation, accelerating the progression of NASH to advanced fibrosis and cirrhosis (28, 29). The WJ-MSCs are a promising therapeutic option due to their differentiation potential and ethical advantages, despite challenges such as potential rejection and limited lifespan. Their therapeutic effects are largely attributed to their paracrine function through exosomes, which transport proteins and nucleic acids that regulate signaling pathways and facilitate tissue regeneration (33). Polyinosinic: Polycytidylic acid exhibits preventive and antifibrogenic effects in liver fibrosis, primarily associated with increased IFN-γ levels. It induces HSC death, causes cell cycle arrest, and enhances NK cell cytotoxicity (34, 35). While the precise mechanisms and anti-inflammatory properties of poly I:C are still not fully understood, it is believed to modulate the immune response through its interaction with Toll-like receptor 3 (TLR3), leading to the secretion of cytokines that influence both the immune microenvironment and fibrosis progression. Importantly, poly I:C may also alter the cargo of exosomes released by MSCs, potentially enriching them with antifibrotic molecules, such as specific microRNAs (miRNAs) or proteins, which can target key signaling pathways like TGF-β/Smad3. This modulation of exosome content could be a critical factor in the observed reduction of HSC activation and ECM deposition, highlighting the potential of poly I:C-stimulated MSC-derived exosomes as a novel therapeutic approach for liver fibrosis (36). Our study investigates the effects of poly I:C-activated MSC exosomes on collagen I and α-SMA expression, as well as SMAD3 phosphorylation, in LX2 cells with cholesterol accumulation. The results highlight the therapeutic efficacy of these exosomes in influencing critical fibrosis pathways, providing valuable insights for the management of liver fibrosis.
In our study, we characterized the cell surface indicators of WJ-MSCs through flow cytometry, confirming the absence of hematopoietic stem cells and monocyte macrophages, while detecting the presence of CD44 and CD105 — markers commonly associated with MSCs. In contrast, Urvi et al. (37) utilized a different panel of markers, highlighting CD105 and CD90, while noting the lack of CD45 and CD34. This difference in marker selection (CD44 in our study versus CD90 in theirs) underscores variability in experimental design and research objectives. Nonetheless, both studies agree on the expression of CD105 as a critical MSC marker. The absence of expression of CD34 and CD45 aligns with the expected MSC phenotype, as these markers are typically linked to hematopoietic stem cells. Our findings also demonstrated the differentiation potential of WJ-MSCs into osteocytes and adipocytes, supported by Alizarin Red and Oil Red O staining. While the comparative study did not detail specific differentiation outcomes, the expression of CD90 and CD105 suggests the multipotent capabilities of MSCs, which include osteogenic and adipogenic differentiation potential.
Our research revealed that cholesterol treatment significantly elevated the mRNA expression levels of TGF-β, α-SMA, and collagen1 genes in LX2 cells, indicating the development and progression of liver fibrosis. This result aligns with the conclusions of Rashidi et al. (38), which also reported increased expression of these fibrosis-related genes following cholesterol exposure, highlighting the critical role of cholesterol in activating HSCs. Notably, treatment with unstimulated exosomes resulted in a substantial reduction in the expression of these genes, suggesting their potential therapeutic role in mitigating fibrosis, which aligns with the comparative study showing that exosomes derived from WJ-MSCs significantly decreased TGF-β, α-SMA, and collagen1α expression. Furthermore, our study revealed that poly I:C-stimulated exosomes exhibited an even greater reduction in TGF-β and α-SMA levels, indicating their enhanced therapeutic potential compared to unstimulated exosomes. These findings suggest that stimulated exosomes may offer a promising strategy to amplify the protective effects against cholesterol-induced liver fibrosis. Overall, the consistency between our results and those of prior studies emphasizes the significant influence of cholesterol on fibrosis development and underscores the therapeutic promise of exosomes in the management of liver fibrosis.
In our study, we examined the therapeutic effects of exosomes on the mRNA expression of NOX1, NOX2, and NOX4 genes in LX2 cells exposed to 100 μM cholesterol for a 24-hour period. We discovered that unstimulated exosomes significantly decreased NOX gene expression, while poly I:C-stimulated exosomes led to an even more pronounced reduction, particularly for NOX1 and NOX4, in fibrotic cells. This aligns with the findings of Afarin et al. (17), which indicated that TGF-β1 treatment increased NOX1, NOX2, and NOX4 expression in LX2 cells. Subsequent treatment of TGF-β1-activated HSCs with exosomes derived from WJ-MSCs at concentrations of 40 and 50 μg/mL resulted in a significant reduction in NOX gene expression after 24 hours. Both studies highlight the healing capabilities of exosomes in mitigating NOX gene expression, which is vital for the progression of fibrosis. The rise in NOX expression due to TGF-β1 treatment parallels our findings related to cholesterol, suggesting that both factors activate similar fibrogenic pathways.
Our study investigated the influence of exosome treatment on the expression of NOX genes in LX2 cells stimulated with cholesterol. The results demonstrated that both unstimulated and poly I:C-stimulated exosomes significantly reduced the expression of NOX1 and NOX4 genes. These results align with the Asadizade et al. (39) study, which reported increased NOX expression following TGF-β1 treatment and a subsequent decrease upon treatment with WJ-MSC-derived exosomes. Additionally, our research showed that cholesterol treatment resulted in a significant increase in ROS production in LX2 cells, whereas exosome treatment led to a notable reduction in ROS levels. These results align with the findings of Asadizade et al., which highlighted the antioxidant effects of curcumin. Together, these studies underscore the therapeutic potential of exosomes and curcumin in mitigating oxidative stress and liver fibrosis progression (39). Overall, both investigations highlight the importance of exosomes and curcumin as promising therapeutic strategies for managing oxidative stress and treating liver fibrosis.
In our study, exosomes derived from WJ-MSCs significantly inhibited the activation of HSCs via the TGF-β/Smad3 pathway. Notably, treatment with these exosomes resulted in a marked reduction in Smad3 phosphorylation, a key mediator in this pathway, as demonstrated by Western blot analysis. This finding suggests a promising therapeutic potential for WJ-MSC-derived exosomes in mitigating liver fibrosis, particularly in the context of cholesterol-induced damage. Additionally, the study by Urvi et al. similarly found that curcumin inhibits Smad3 phosphorylation in TGF-β-activated HSCs, reinforcing the notion of its antifibrotic properties. While both WJ-MSC-derived exosomes and curcumin target the same signaling pathway, they differ in their mechanisms of action; curcumin acts as a small molecule inhibitor, while WJ-MSC-derived exosomes operate as biological entities (37). This distinction highlights their unique therapeutic strategies, as curcumin demonstrates concentration-dependent effects, whereas WJ-MSC-derived exosomes exhibit efficacy at lower concentrations over shorter incubation times. Overall, both approaches underscore the critical role of the TGF-β/Smad3 pathway in liver fibrosis and present valuable options for therapeutic intervention.
Rashidi et al. further corroborate the inhibitory effect of exosomes derived from WJ-MSCs on Smad3 phosphorylation in HSCs treated with TGF-β (38). In contrast to our study, which focused on cholesterol-induced damage, Rashidi et al. employed a direct TGF-β challenge. Their results showed that exosomes significantly reduced Smad3 phosphorylation across various concentrations, highlighting the versatility and robustness of exosomal intervention (38). This study complements our findings by providing additional evidence that exosomes can counteract TGF-β-induced cellular responses, reinforcing the potential of exosomal therapies in diverse pathological contexts.
Collectively, these studies and our results present a consistent narrative where both natural compounds, such as curcumin, and biological products, like WJ-MSC exosomes, can effectively disrupt the TGF-β/Smad3 cascade, which is pivotal for HSC activation and the advancement of liver fibrosis. This comparison underscores the diverse strategies available for modulating this pathway and suggests the potential for integrative approaches that combine the advantages of small molecules and bioengineered products in the treatment of hepatic fibrosis. However, it is important to note that our study was conducted in vitro, and there were no specific eligibility criteria or control interventions, which should be considered in future studies. Additionally, while the study provides promising results, further research is required to evaluate the efficacy of exosome-based therapies in in vivo models and clinical settings, where factors such as bioavailability, delivery mechanisms, and long-term effects may influence therapeutic outcomes.
5.1. Conclusions
Our study demonstrates that exosomes derived from WJ-MSCs, particularly when stimulated with poly I:C, have significant therapeutic potential in modulating liver fibrosis pathways. Cholesterol accumulation in NASH patients leads to HSC activation, excessive ECM synthesis, and oxidative stress, primarily driven by TGF-β and NOX enzymes. Our results indicate that WJ-MSC-derived exosomes can inhibit Smad3 phosphorylation and the TGF-β/Smad pathway, thereby reducing fibrosis-related gene expression and oxidative stress. The enhanced efficacy of stimulated exosomes compared to unstimulated ones highlights the importance of exosome activation in therapy optimization. Overall, this research underscores the multifaceted nature of liver fibrosis and the promising function of WJ-MSC-derived exosomes in its management, paving the way for future therapeutic applications.