1. Context
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent hepatic pathology globally and has the potential to progress into non-alcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma (HCC) (1). The global prevalence of NAFLD is 32.4% (2). Approximately 25% to 35% of NASH patients experience liver fibrosis, which is associated with long-term adverse outcomes (2). The severity of liver fibrosis is a significant factor in liver-related mortality in NAFLD. The risk of progression to cirrhosis includes complications such as type 2 diabetes mellitus (T2DM), aging, hypertension, and high BMI, as well as genetic factors including the patatin-like phospholipase domain-containing protein 3 (PNPLA3) I148M variant (3-5). It is projected that NAFLD will become the primary indication for liver transplantation and a significant burden of HCC in the coming decades (6).
2. Objectives
Given the increasing global prevalence and the lack of definitive treatments for NASH or a first-line treatment for NAFLD, many researchers are exploring cures for NASH in individuals with NAFLD through Precision Gene Therapy. Through a comprehensive analysis of eligible published studies, our meta-analysis and systematic review aim to summarize the current evidence on the prevalence of the rs738409 GG homozygous variant and its association with the risk of fibrosis in patients with biopsy-confirmed NAFLD. The findings can support the data basis for Precision Gene Therapy of NAFLD.
3. Methods
3.1. Search Strategy
In accordance with the PRISMA guidelines (7), we systematically identified published studies by reviewing PubMed, Embase, and the Cochrane database from their inception to July 3, 2024, through reference tracing of retrieved articles. Keywords for the search were as follows: ("PNPLA3", "rs738409 gene or variants or polymorphism or alleles") and ("Non-alcoholic Fatty Liver Disease", "NAFLD"). After removing duplicate studies, two investigators independently reviewed the titles and abstracts of the remaining articles to assess their eligibility based on the following inclusion criteria: (1) Studies published in the English language; (2) studies reporting sufficient data concerning the rs738409 GG homozygous on NAFLD that could be extracted for analysis; (3) study populations representative of the general population and prospective cohort studies or retrospective case-control studies; (4) studies that stated the definition of NAFLD (8); (5) studies reporting data on NAFLD in humans and focusing on adults and adolescents.
3.2. Exclusion Criteria
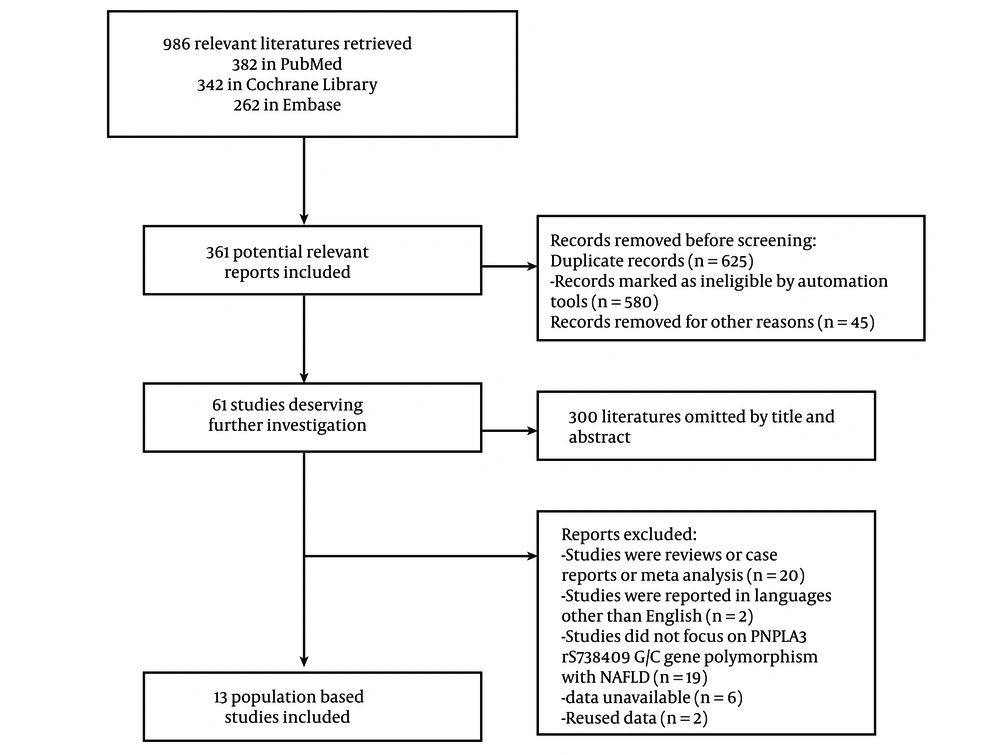
The detailed exclusion criteria are shown in Figure 1. Articles were discussed further until consensus was reached in cases of disagreement between authors.
3.3. Data Extraction
Two investigators independently extracted information from each eligible article. Recorded data included the name of the first author, year of publication, study location (country), number of participants, number of GG, GC, CC homozygous proportion of NAFLD, gender and number of patients and controls, method of NAFLD ascertainment, and the stage of fibrosis in different homozygous of PNPLA3. If there was exposure classification in categorical and continuous manners, we used the former. We treated the results as two independent groups, separating adults and adolescents, if age stratification was applied. The same reviewers independently assessed the quality of cohort studies with the Newcastle-Ottawa Scale (9). At each stage, the reviewers compared their results and resolved any disagreements through consensus. We used data from the latest publication if multiple articles for a single study were found.
3.4. Statistical Analysis
The exposure of interest, consistently reported by at least two studies, was included in a meta-analysis and systematic review. All studies were pooled to generate a combined effect size and 95% confidence interval (CI) for each outcome, with estimates of relative risk (RR). Heterogeneity between studies was assessed using the I2 statistic, with a cut-off of 50%, and the χ2 test, with a P-value < 0.10 (10). If a statistically significant degree of heterogeneity was found (P < 0.10), it was further analyzed. When heterogeneity could not be explained, the random effects model, which accounts for variation both within and between studies, was used to combine the results (10, 11).
4. Results
4.1. Study Selection and Characteristics
Three databases yielded a total of 986 results: Three hundred and eighty-two from PubMed, 342 from the Cochrane Library, and 262 from Embase. Deduplication in EndNote removed 580 results, and an additional 45 duplicates were removed for other reasons. A further 300 results were excluded for not meeting the inclusion criteria during the title and abstract review. Finally, 61 articles were reviewed in full, and 13 articles were included in this study (12-24). The detailed exclusion criteria are shown in Figure 1. The key characteristics of the included articles are presented in Table 1. Of the 13 eligible studies published between 2010 and 2022, 10 were adult studies and 3 were underage studies. Eleven studies were conducted in Asia, while the other two studies were from Austria and Germany, focusing on non-Hispanic whites. The included studies declared that NAFLD was diagnosed by biopsy-proven methods. Data on the rs738409 GG homozygous in fibrosis were available in 7 eligible studies that reported data for liver fibrosis. Regarding the assessment of quality, 90.0% (n = 12) of the studies were rated as moderate in quality, with none being rated as low.
| Author (y) | Ethnicity | Diagnosis | NAFLD | CC/CG/GG | Fibrosis | |||
|---|---|---|---|---|---|---|---|---|
| No Fibrosis | Perisinusoidal Fibrosis | Periportal Fibrosis | Bridging Fibrosis | |||||
| CC/CG/GG | CC/CG/GG | CC/CG/GG | CC/CG/GG | |||||
| Targher et al. (2019) (12) | Children and adolescents | Biopsy-proven | 142 | 41/56/45 | 8/10/1 | 27/40/30 | 6/6/14 | 0/0/0 |
| Li et al. (2022) (13) | Chinese individuals | Biopsy-proven | 523 | 144/252/127 | (F ≥ 2 stage) 22/49/31 | |||
| Paternostro et al. (2021) (14) | Austria and Germany | Biopsy-proven | 703 | 358/267/78 | ||||
| Nobili et al. (2019) (17) | Children and adolescents | Biopsy-proven | 328 | 101/141/86 | ||||
| Xu et al. (2022) (15) | Chinese Wenzhou | Biopsy-proven | 415 | 126/190/99 | (F ≥ 2 stage) CC + CG/GG:69/20 | |||
| Seko et al. (2017) (16) | Japanese patients | Biopsy-proven | 238 | 33/110/95 | Fibrosis stage (0/1/2/3/4):CC + CG/GG:(14/20/8/7/1)/ (3/12/9/8/4) | |||
| Idilman et al. (2020) (18) | Turkey | Biopsy-proven | 174 | 48/43/83 | (F ≥2 stage)GG genotype (25/83); GC + CC:12/91 | |||
| Zain et al. (2012) (19) | 54 Chinese, 31 Indians, and 59 Malays | Biopsy-proven | 144 | 48/63/33 | ||||
| Uygun et al. (2017) (20) | Turkey | Biopsy-proven | 216 | 64/90/62 | ||||
| Seko et al. (2020) (21) | Japanese patients | Biopsy-proven | 290 | 49/119/122 | Fibrosis stage (0/1/2/3/4): CC + CG/GG: (51/59/27/19/12)/ (30/28/23/29/12) | |||
| Valenti et al. (2010) (22) | children and adolescents Italian | Biopsy-proven | 149 | 65/61/23 | ||||
| Nan et al. (2021) (6) | China | Biopsy-proven | 59 | 12/27/20 | ||||
| Vilar-Gomez et al. (2021) (24) | Non-Hispanic whites | Biopsy-proven | 452 | 128/210/114 | Significant fibrosis (F ≥ 2):52/105/62 | |||
Summary of Key Characteristics of Included Studies
4.2. Meta-Analysis
4.2.1. The Proportion of the rs738409 GG Homozygous in Patients with Biopsy-Proven Non-alcoholic Fatty Liver Disease
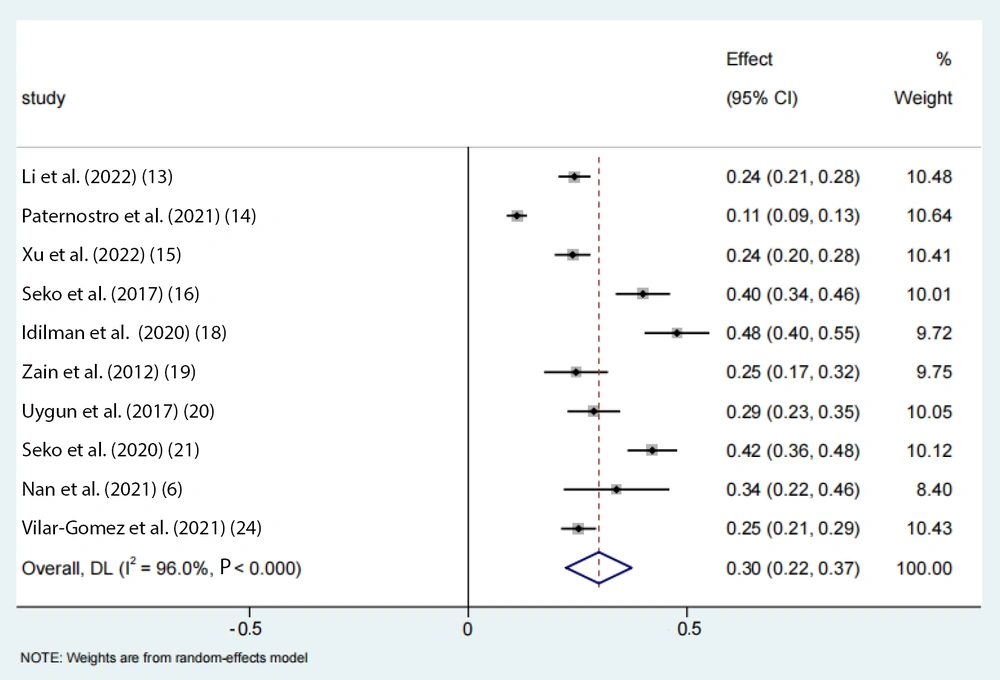
A total of 13 eligible studies, encompassing 3,823 individuals, were included in the analysis. The pooled proportion of PNPLA3 rs738409 GG homozygous individuals with NAFLD was estimated to be 30% (95% CI: 0.22 - 0.37, I2 = 96%, P < 0.01) in adults (Figure 2).
4.2.2. The Proportion of the rs738409 GG Homozygous in Patients with Non-alcoholic Fatty Liver Disease with Advanced Fibrosis (≥ 2)
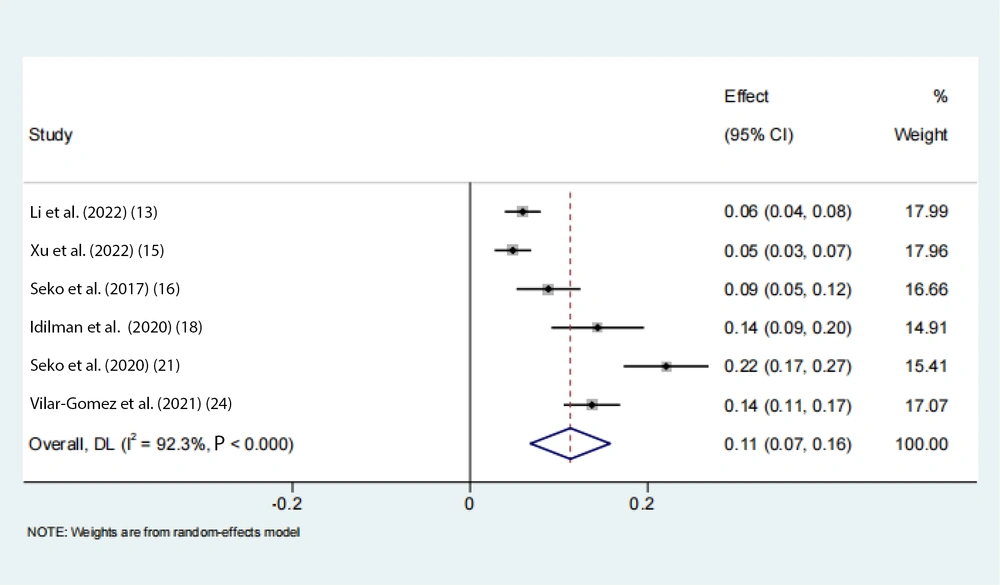
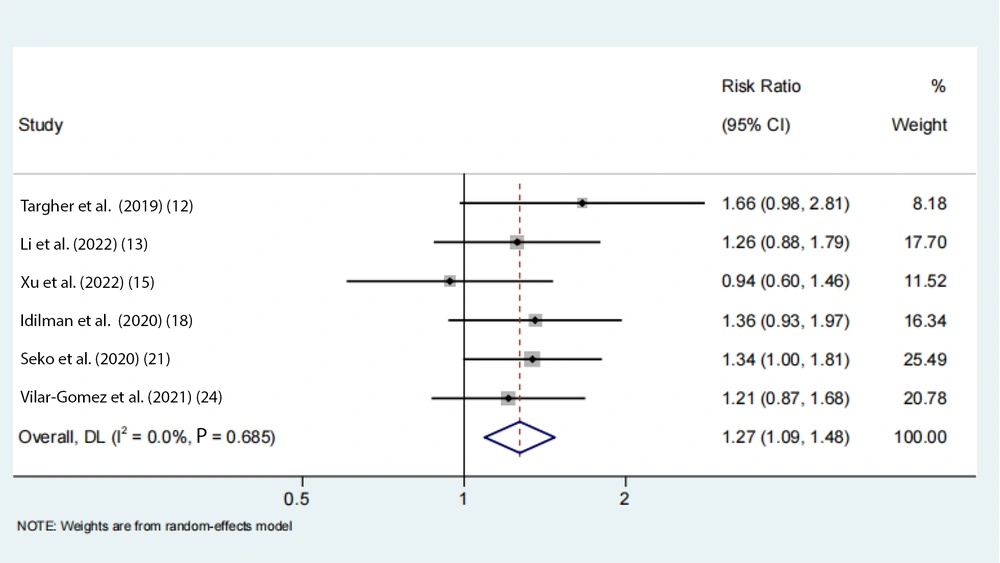
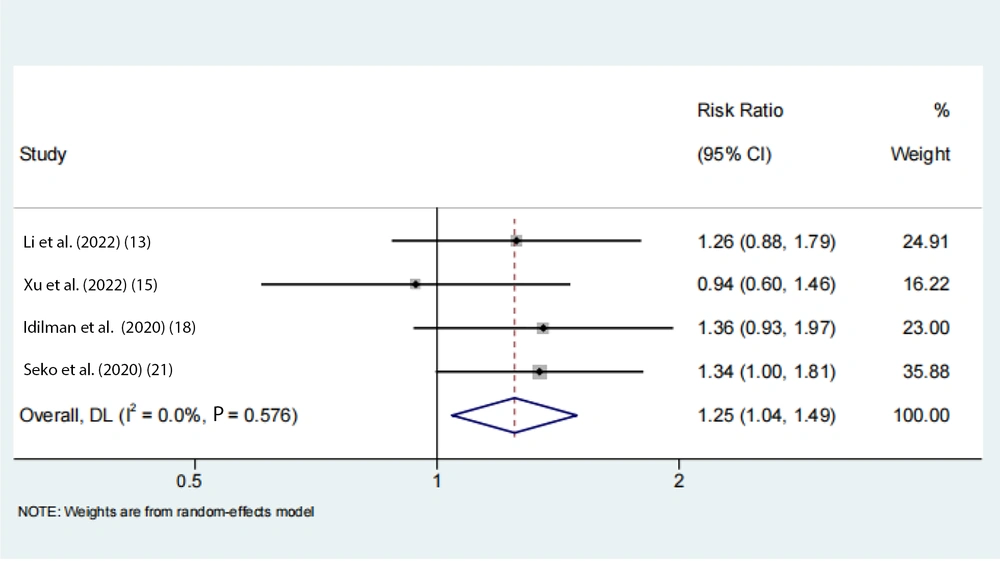
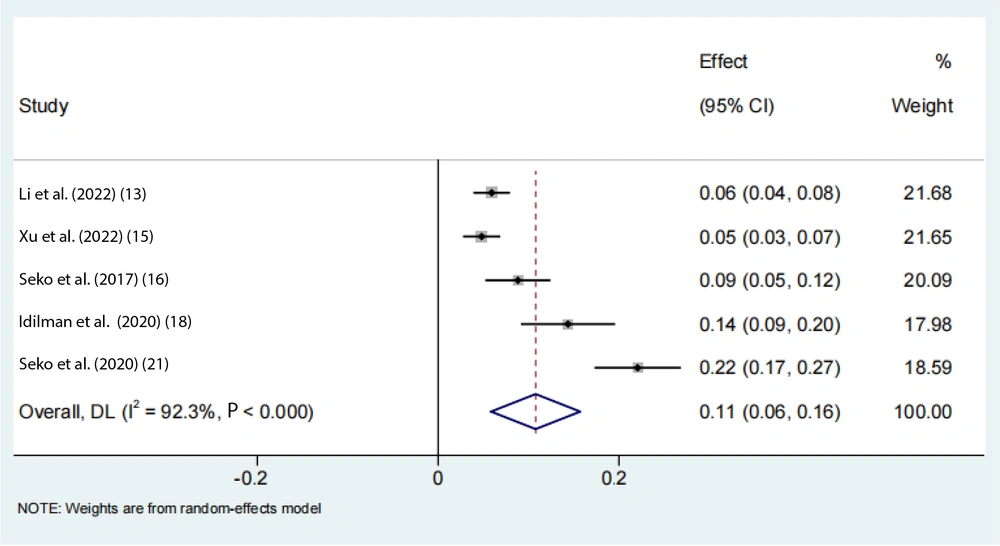
Data on the rs738409 GG homozygous variant in fibrosis were available from 7 eligible studies. Advanced fibrosis (≥ 2) was estimated to be 11% (95% CI: 0.07 - 0.16, I2 = 92.3%, P < 0.01) in adults (Figure 3). Two articles from the Chinese population showed that advanced fibrosis (≥ 2) was estimated to be 5.9% and 4.8%. Our results indicated that the incidence of GG homozygous individuals in patients with advanced fibrosis (≥ 2) had a 1.27-fold and 1.24-fold greater proportion compared with those with low-level fibrosis (< 2) in the adult Asian population, respectively (Figures 4 and 5).
4.3. Subgroup Analyses
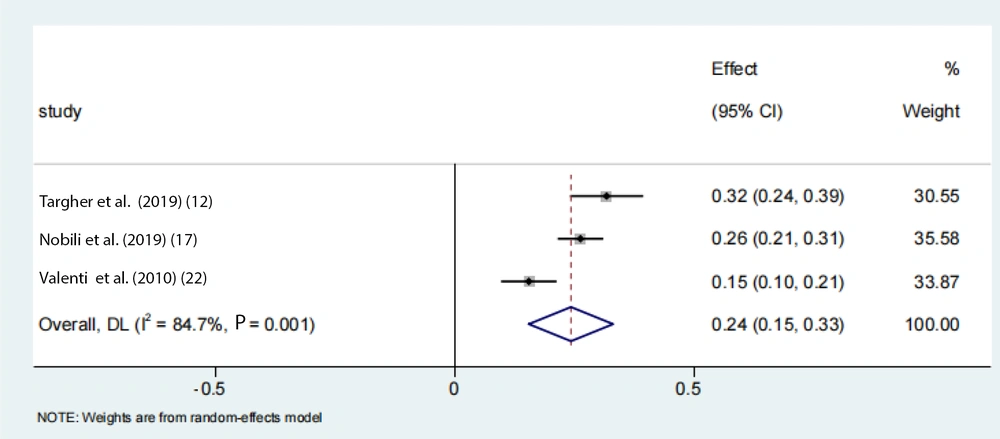
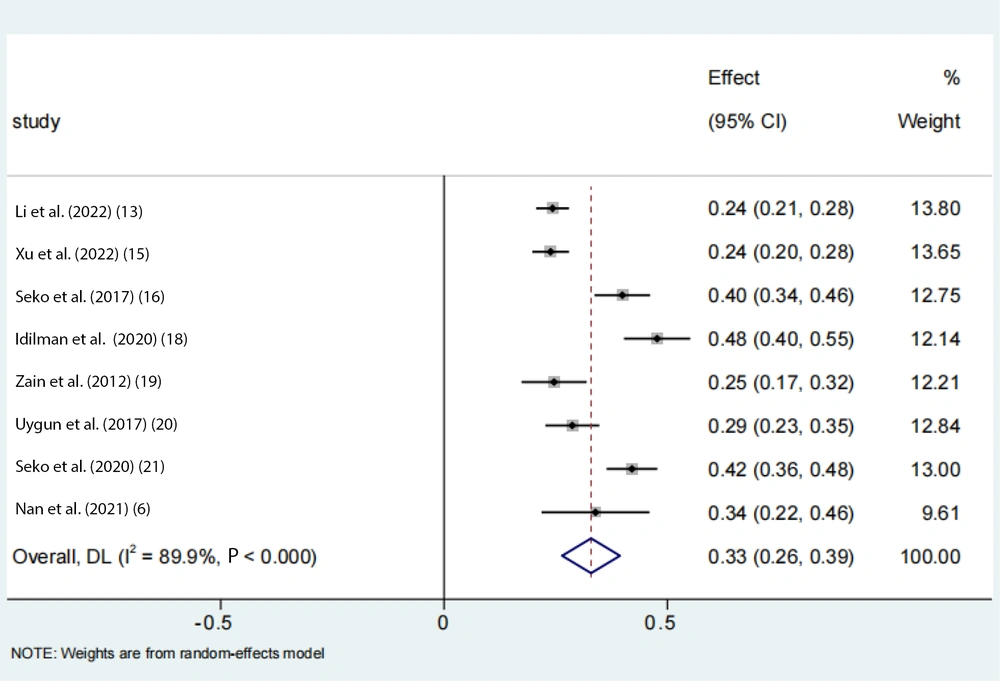
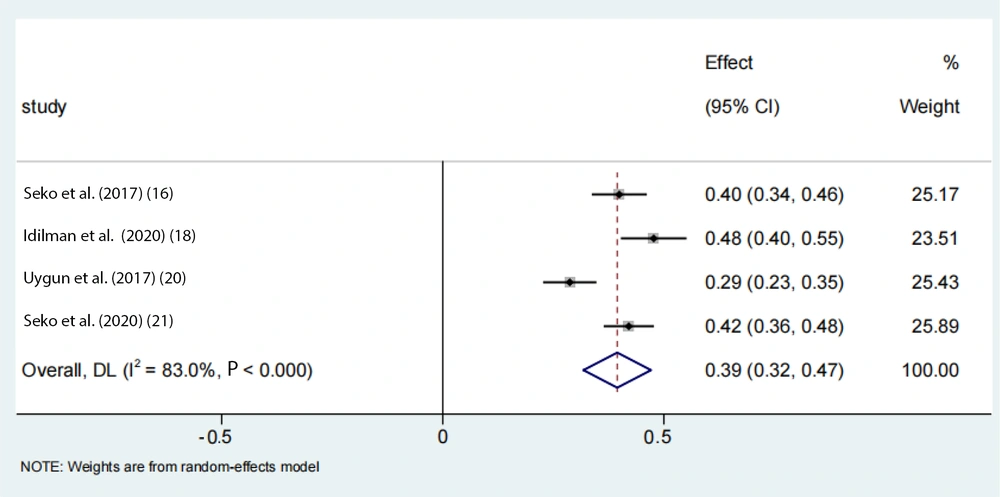
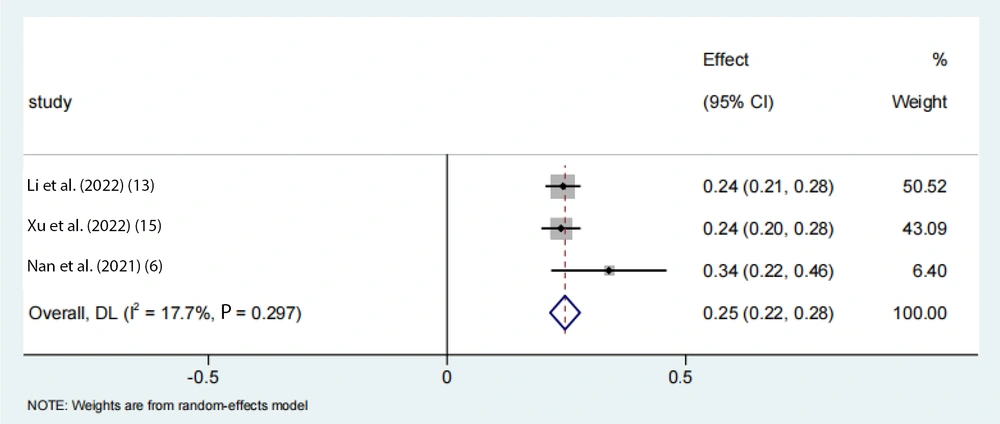
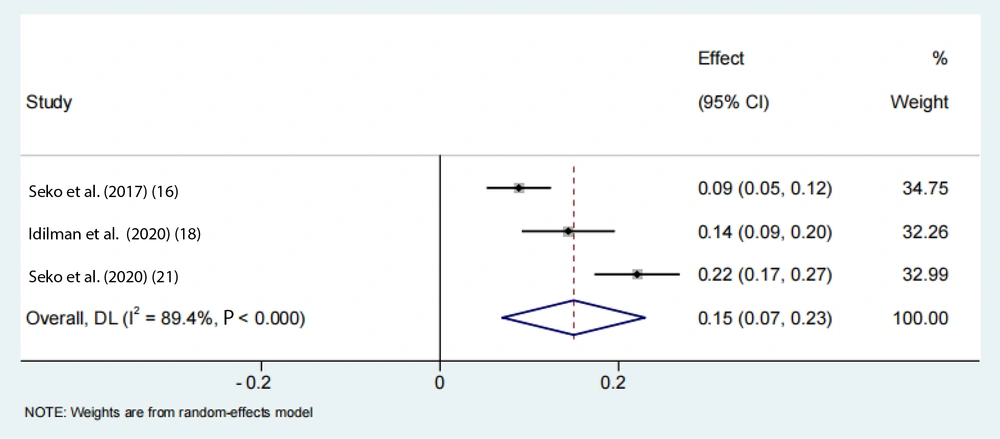
To account for the moderate to severe heterogeneity observed among the studies, a subgroup analysis was conducted. The pooled proportion of PNPLA3 rs738409 GG homozygous individuals with NAFLD was estimated to be 24% (95% CI: 0.15 - 0.33, I2 = 84.7%, P < 0.01) in adolescents, 33% (95% CI: 0.26 - 0.39, I2 = 89.9%, P < 0.01) in the Asian population, 39% (95% CI: 0.32 - 0.47, I2 = 83%, P < 0.01) in Japanese individuals, and 25% (95% CI: 0.22 - 0.28, I2 = 17.7%, P < 0.01) in Chinese individuals (Figures 6 -9). Advanced fibrosis (≥ 2) was estimated to be 11% (95% CI: 0.06 - 0.16, I2 = 92.3%, P < 0.01) in the Asian population and 15% (95% CI: 0.07 - 0.23, I2 = 89.4%, P < 0.01) in Japanese individuals (Figures 10 and 11).
4.4. Sensitivity Analyses and Publication Bias Tests
A sensitivity analysis was conducted to test the data reliability of the results. When the studies were removed one by one, the findings showed that the pooled outcomes remained unchanged, indicating that our results are stable and reliable. The outcomes generated by the fixed-effect model were consistent with those from the random-effects model. Furthermore, Egger’s tests were conducted to evaluate publication bias, with the results showing no statistically significant bias.
5. Discussion
Our research estimated the proportion of the rs738409 GG homozygous variant and its associated risk of fibrosis. We reported three main findings. First, the currently available data suggest varying incidence rates of GG homozygous individuals in NAFLD and NAFLD-related liver fibrosis populations across different regions and countries. Second, the proportion of the rs738409 GG homozygous variant differs among countries and specific populations. Third, our findings highlight the need for genetic screening in NAFLD patients to identify high-risk individuals for early intervention and targeted therapies.
Previous studies indicate that the global prevalence of NAFLD is 38% (25). Nonalcoholic fatty liver disease is linked to a range of detrimental clinical outcomes that can ultimately increase mortality, including severe hepatic inflammation and fibrosis, metabolic and cardiovascular diseases, and extrahepatic cancers (26, 27). Recent studies have shown a strong association between NAFLD and heart failure (HF). In propensity score-matched cohorts, NAFLD was associated with a 2.52-fold higher risk of HF (28). The number of cases and liver-related deaths is expected to rise significantly by 2030 (29). Many studies suggest a strong link between PNPLA3 I148M and NAFLD mortality, with other factors such as lifestyle habits, age, sex, obesity, and T2DM also playing a role (30-32). However, the potential synergistic effects between this genetic variant and environmental exposures have not been extensively investigated (33).
Phospholipase domain-containing protein 3 rs738409 is a non-synonymous mutation where cytosine is replaced by guanine, resulting in the substitution of isoleucine with methionine at the 148th position of the protein (I148M) (34). A substantial body of evidence suggests that carriage of the PNPLA3 I148M polymorphism, characterized by the presence of the G allele with marked variation in mutation rates among ethnic populations, is the most important feature of higher histologic steatosis (35). The NAFLD patients carrying the G allele are characterized by the activation of hepatic stellate cells (HpSC), which is associated with more aggressive histological patterns, such as portal fibrosis, and increased oxidative stress (36). Patients with the homozygous variant (GG) have an increased risk of disease progression, liver-related events, and HCC (36). This finding has made the PNPLA3 I148M polymorphism a hot spot in current NAFLD drug development.
Furthermore, in a general US population sample, the association between PNPLA3 I148M and liver-related and all-cause mortality has increased, highlighting the need for effective targeted treatments for NAFLD patients with the PNPLA3 I148M mutation to reduce disease progression and death in carriers (37). Banini et al. succeeded in introducing the I148M mutation into the mouse gene and establishing a steatohepatitis phenotype, finding that under conditions of a high-sugar, high-fat diet, PNPLA3 I148M could accelerate NAFLD progression to NASH and fibrosis. This gene-environment interaction leads to metabolic changes, primarily affecting polyunsaturated fatty acid (PUFA) and sphingolipid metabolic pathways, and activates inflammatory and cell damage pathways through STAT3 downstream (38). Silencing PNPLA3 I148M with siRNA to inhibit the activation of STAT3 is also one of the research directions for the future (39).
In conclusion, PNPLA3 plays an important role in the process of liver fibrosis in NAFLD patients, and this protein is an attractive target for the treatment of NAFLD (40). Therefore, this meta-analysis helps us understand the proportion of this polymorphism in different populations and its impact on the progression of NAFLD, thereby bringing more effective treatment strategies for NAFLD patients.
Our study indicated the proportion of the rs738409 GG homozygous variant in adults, adolescents, the Asian population, Japanese, and Chinese individuals, and the risk of fibrosis in patients from different countries and regions. However, given the limitations of our study, these findings should be interpreted with caution. Firstly, all included studies were published articles, and data from other sources could potentially influence the results. Secondly, the wide CIs in some estimates for Japanese populations suggest a small sample size or study variability. Future studies are needed to obtain more accurate results. Thirdly, the moderate to severe heterogeneity observed among the studies was not fully accounted for. Larger-scale studies are needed in the future to validate these findings.
5.1. Conclusions
In conclusion, our findings suggest that the rs738409 GG homozygous proportion in biopsy-proven NAFLD varies due to geographic and population heterogeneity. Our results indicate that the rs738409 GG homozygous variant exerts a strong influence on the development of fibrosis. These findings underscore the importance of genetic screening in NAFLD patients to identify high-risk individuals for early intervention and targeted therapies.