1. Background
Primary liver cancer, which arises in the liver from hepatocytes or intrahepatic biliary epithelial cells, is considered one of the most prevalent and aggressive types of tumors globally (1). According to the 2022 Global Cancer Burden Report by the International Agency for Research on Cancer (IARC), liver cancer is the sixth most common and third deadliest cancer worldwide (2). In 2022, approximately 865,000 new liver cancer cases and 758,000 deaths were estimated globally, comprising 4.3% and 7.8% of all cancer cases and deaths related to malignant tumors, respectively (3). Regions such as East Asia, Southeast Asia, and North Africa have high incidence rates of liver cancer, with China bearing a significant portion of the burden, accounting for 45% of the world’s new liver cancer cases, amounting to approximately 389,000 cases (4). Hepatocellular carcinoma constitutes the primary type of liver cancer, comprising between 85% to 90% of all cases (5).
Surgical resection is considered the primary treatment option for liver cancer; however, postoperative recurrence remains a crucial factor impacting its effectiveness (6). As stated in the "2020 CSCO Guidelines for the Diagnosis and Treatment of Primary Liver Cancer", the recurrence rate within five years following surgical resection for liver cancer can be as high as 50% to 70% (7). Recurrence after surgery is categorized into early and late stages, with early recurrence associated with high-risk factors such as microvascular invasion (MVI), non-anatomic resection, large tumor size (diameter > 5 cm), residual micrometastases, and serum AFP > 32 ng/mL, among others (8, 9). Therefore, effective prophylactic interventional therapy is particularly important for liver cancer patients at high risk of early recurrence.
Transarterial chemoembolization (TACE) is a standard treatment for liver cancer that has gained widespread adoption in clinical practice due to its distinctive therapeutic mechanism and generally reliable safety characteristics (10). The principle behind TACE involves delivering chemotherapeutic drugs and embolic agents precisely to the arteries supplying blood to the tumor through a catheter. The chemotherapeutic agents directly kill the tumor cells, while the embolic agents block the blood supply to the tumor, inhibiting tumor growth and promoting tumor cell apoptosis (11, 12). Transarterial chemoembolization avoids the severe adverse reactions associated with systemic chemotherapy to some extent, effectively controlling the tumor while ensuring improved quality of life for patients, making TACE a significant treatment option for those with primary liver cancer (13, 14).
The blood supply to primary liver cancer is abundant and complex, with not only the main hepatic artery but also potential multiple arterial supplies or collateral circulations, such as extrahepatic collateral arteries, nourishing the tumor (15). Especially for larger tumors or tumors in special locations, it is challenging to completely embolize all tumor-feeding arteries through TACE (16). The residual feeding arteries continue to provide nutrients to the tumor cells, preventing complete tumor inactivation and increasing the risk of tumor recurrence and metastasis. A single TACE embolization rarely achieves complete tumor necrosis, and residual cancer cells may acquire enhanced invasiveness and proliferative capacity in a hypoxic environment, accelerating tumor growth and metastasis. The TACE treatment can impose a significant burden on the patient’s liver function, particularly for those with coexisting hepatitis or cirrhosis, as repeated embolizations may lead to liver function impairment, affecting long-term outcomes. Furthermore, the long-term efficacy of TACE is not ideal, with high risks of recurrence and metastasis, and the emergence of primary or secondary drug resistance is possible. In terms of adverse reactions, TACE treatment may induce post-embolization syndrome, including fever, pain in the liver area, nausea, vomiting, and abdominal distension, which typically last for 1 - 7 days (17). Additionally, complications such as leukopenia, liver function impairment, kidney function impairment, and bleeding at the puncture site may occur (18). In some cases, severe issues such as gallbladder necrosis or damage to the renal or splenic arteries due to inadvertent embolization may also arise (19). Therefore, the search for a more effective prophylactic interventional treatment method is particularly important.
In recent years, hepatic arterial infusion chemotherapy (FOLFOX-HAIC) has emerged as a new treatment method for liver cancer, gradually gaining attention from clinicians. The FOLFOX-HAIC involves the continuous infusion of chemotherapeutic agents (such as those comprising the FOLFOX regimen, including oxaliplatin and fluorouracil) through the hepatic artery, maintaining a high local drug concentration in the liver for an extended period. Such a sustained high-concentration drug environment can more effectively kill tumor cells, particularly exerting a stronger inhibitory effect on microscopic lesions and potential cancer cells (20, 21). Although FOLFOX-HAIC also utilizes chemotherapeutic agents, the drugs are delivered slowly and continuously to the liver, resulting in relatively less systemic inflammatory stimulation and milder systemic adverse reactions in patients (22). For some special types of primary liver cancer, such as giant liver cancer (with a large tumor diameter) or tumors with multiple feeding arteries, TACE often finds it difficult to completely embolize all the feeding arteries, leading to a higher risk of tumor residue and recurrence. In contrast, FOLFOX-HAIC is not limited by the complexity of the tumor’s blood supply vessels and can comprehensively kill the tumor by continuously infusing chemotherapeutic agents. Even if there are multiple feeding arteries, the drug can reach all parts of the tumor, thereby improving treatment efficacy (23). A phase III study led by Professor Shi Ming’s team in 2022 showed that HAIC-FOLFOX had a higher overall response rate (45.9% vs. 17.9%), a higher conversion rate to surgery (23.9% vs. 11.5%), a longer overall survival (OS) (23.1 months vs. 16.07 months), and a longer progression-free survival (PFS) (9.63 months vs. 5.4 months) compared to TACE for the treatment of large liver cancer (with the largest tumor lesion ≥ 7 cm) (24).
2. Objectives
The present study aims to further verify the superiority of FOLFOX-HAIC by comparing its clinical application effects with those of TACE in prophylactic interventional therapy after resection of primary liver cancer.
3. Methods
3.1. Clinical Data
This study employed the intention-to-treat (ITT) analysis approach. This retrospective clinical study enrolled 60 patients diagnosed with primary liver cancer at Dongyang People’s Hospital who underwent surgical treatment between January 2022 and December 2024. The hospital has extensive experience in treating primary liver cancer and offers both surgical and interventional treatment options. The study was approved by the hospital’s ethics committee (YZ20210618). Patients who met the inclusion criteria were identified from the hospital’s surgical records. They were approached by the study team during their postoperative follow-up visits and provided with detailed information about the study. Although this was a retrospective cohort study, we ensured that the treatment allocation (TACE or FOLFOX-HAIC) was based on clinical indications and patient preferences at the time of treatment decision. The study design aimed to reflect real-world clinical practice. We conducted a thorough review of medical records and imaging reports to ensure the accuracy and completeness of the data. Any discrepancies or missing data were resolved through additional chart reviews or consultations with the treating physicians.
3.2. Eligibility Criteria
3.2.1. Inclusion Criteria
Patients with Child-Pugh class A or B liver function capable of tolerating surgery well; complete tumor resection during surgery with pathological examination confirming primary liver cancer and no tumor cell residue at the surgical margins; voluntary receipt of TACE or FOLFOX-HAIC 1 to 3 months after surgical resection, with no tumor vessels or tumor staining identified on arteriography.
3.2.2. Exclusion Criteria
Patients with severe hepatic dysfunction (Child-Pugh class C), obvious jaundice, hepatic encephalopathy, refractory ascites, or hepatorenal syndrome; severe coagulation dysfunction that cannot be corrected; concurrent active hepatitis or severe infection that cannot be treated simultaneously; cachexia or multi-organ failure; significant reduction in peripheral blood WBC and PLT counts with no significant improvement after medical intervention; severe renal dysfunction; and other severe conditions such as alcoholism, drug abuse, or mental illness that prevent the patient from cooperating.
3.2.3. Withdrawal Criteria
Intrahepatic tumor progression or distant metastasis detected before the first prophylactic interventional treatment after liver cancer surgery; development of severe non-oncological diseases making it impossible to continue treatment or routine follow-up; patient’s voluntary request to withdraw from follow-up.
3.3. Grouping Criteria and Treatment Method
Based on their treatment methods, patients were divided into two groups: Transarterial chemoembolization group and the FOLFOX-HAIC group, with 30 patients in each. Prophylactic interventional therapy via the hepatic artery was scheduled 1 - 3 months after surgical resection for all patients. The interventional procedures were performed on a DSA machine (Philips). Percutaneous puncture of the femoral artery (or other arteries including the radial artery or subclavian artery) was carried out using the Seldinger technique. Initially, a 5 F catheter was inserted into both the superior mesenteric artery and the celiac trunk to conduct the routine angiography procedure. After confirming the absence of tumor vessels and lesions, a 2.7 F microcatheter was used for superselective transarterial interventional therapy via the hepatic artery, using either TACE or FOLFOX-HAIC. For TACE, a chemoembolization emulsion was prepared by mixing the chemotherapeutic agent epirubicin 30 mg with 2 mL of lipiodol and injected through the catheter. For FOLFOX-HAIC, first, 400 mg/m2 of fluorouracil is rapidly injected through the hepatic artery. Subsequently, 400 mg/m2 of leucovorin is infused through the hepatic artery over a period of 2 hours. At the same time as the leucovorin infusion begins, 85 mg/m2 of oxaliplatin is also infused over a period of 2 hours. After the completion of the leucovorin and oxaliplatin infusions, a continuous infusion of 2400 mg/m2 of fluorouracil is initiated, lasting for 46 hours.
3.4. Follow-up and Observation Indicators
The follow-up endpoint was determined as the occurrence of intrahepatic lesion recurrence or death. The diagnostic criteria for recurrence were: Any imaging examination such as ultrasound B, CT, MRI, or hepatic arteriography revealing space-occupying lesions in the liver that conform to the characteristics of primary liver cancer, confirming post-resection recurrence. If no space-occupying lesions were found on imaging examinations but the serum AFP level rose again to > 200 μg/L after surgery, and active liver disease or pregnancy were ruled out, it was also considered as recurrence after liver cancer resection. The incidence of adverse reactions after interventional therapy was also recorded for both groups. Follow-up was conducted every 3 months for 2 years post-surgery. A follow-up form was completed at each follow-up visit, and any tumor recurrence, metastasis, or death was recorded on the form.
3.5. Outcomes
The primary endpoint is OS, calculated from the date the patient is randomized to the FOLFOX-HAIC or TACE treatment group. The calculation continues until the date of the patient’s death from any cause. If the patient remains alive during the study period, the calculation is carried out until the date of the last follow-up record. Secondary endpoints include:
- Progression-free survival: The time interval from randomization to the FOLFOX-HAIC or TACE treatment group until disease recurrence assessed by imaging examinations and postoperative serum AFP levels or death due to any cause, whichever occurs first.
- Symptomatic PFS: The time interval from randomization until the patient’s FHSI-8 Questionnaire score increases by 4 points or more from baseline, or the ECOG performance status score reaches 4, or death due to any cause, whichever occurs first. This indicator aims to assess the patient’s quality of life and the impact of the disease on daily activity capabilities.
- Comparison of recurrence rates: Some patients experience recurrence after liver cancer surgery.
- Adverse events (AEs): Evaluated in accordance with the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0). This indicator aims to comprehensively record and analyze the adverse reactions and complications that may occur during and after FOLFOX-HAIC or TACE treatment in patients after primary liver cancer resection.
3.6. Statistical Analysis
Data analysis was conducted using SPSS 25.0. Normally distributed variables were presented as mean ± SD and compared with t-tests. Categorical data were presented as No. (%) and compared with χ2 tests. Survival was analyzed by Kaplan-Meier and compared by log-rank test. A P < 0.05 was considered significant.
4. Results
4.1. Comparison of Baseline Characteristics
In terms of baseline demographic characteristics and clinical indicators, no significant statistical differences were observed between the two groups (P > 0.05), ensuring the comparability of subsequent intervention effects (Table 1).
| Variables | TACE Group (n = 30) | FOLFOX-HAIC Group (n = 30) | χ2/t | P-Value |
|---|---|---|---|---|
| Gender | 0.067 | 0.792 | ||
| Male | 14 (46.67) | 15 (50.00) | ||
| Female | 16 (53.33) | 15 (50.00) | ||
| Age (y) | 54.77 ± 7.49 | 55.47 ± 7.99 | 0.350 | 0.728 |
| Liver function (Child-Pugh) | 0.021 | 0.885 | ||
| A | 8 (26.67) | 9 (30.00) | ||
| B | 22 (73.33) | 21 (70.00) | ||
| Tumor size (cm) | 0.267 | 0.875 | ||
| < 5 | 3 (10.00) | 2 (6.67) | ||
| 5 ~ 10 | 20 (66.67) | 20 (66.67) | ||
| > 10 | 7 (23.33) | 8 (26.67) | ||
| Tumor number | 0.073 | 0.787 | ||
| < 4 lesions | 10 (33.33) | 11 (36.67) | ||
| ≥ 4 lesions | 20 (66.67) | 19 (63.33) | ||
| Excision range | 0.305 | 0.859 | ||
| Resection of right lobe tumor | 12 (40.00) | 14 (46.67) | ||
| Left lobe tumor resection | 13 (43.33) | 12 (40.00) | ||
| Others (middle lobe of liver, caudate lobe) | 5 (16.67) | 4 (13.33) | ||
| Complicated with cirrhosis | 22 (73.33) | 21 (70.00) | 0.082 | 0.775 |
| Cause of liver disease | 0.219 | 0.896 | ||
| Hepatitis B | 26 (86.67) | 27 (90.00) | ||
| Hepatitis C | 3 (10.00) | 2 (6.67) | ||
| Others | 1 (3.33) | 1 (3.33) | ||
| AFP (ng/mL) | 496.77 ± 55.79 | 483.83 ± 61.97 | 0.850 | 0.399 |
| ALT (U/L) | 52.63 ± 8.74 | 53.43 ± 7.56 | 0.379 | 0.706 |
| AST (U/L) | 63.53 ± 7.45 | 64.30 ± 7.87 | 0.387 | 0.700 |
| TBIL (mmol/L) | 15.87 ± 1.98 | 15.33 ± 2.22 | 0.983 | 0.330 |
Comparison of Baseline Characteristics a
4.2. Comparison of Overall Survival Between Hepatic Arterial Infusion Chemotherapy Group and Transarterial Chemoembolization Group
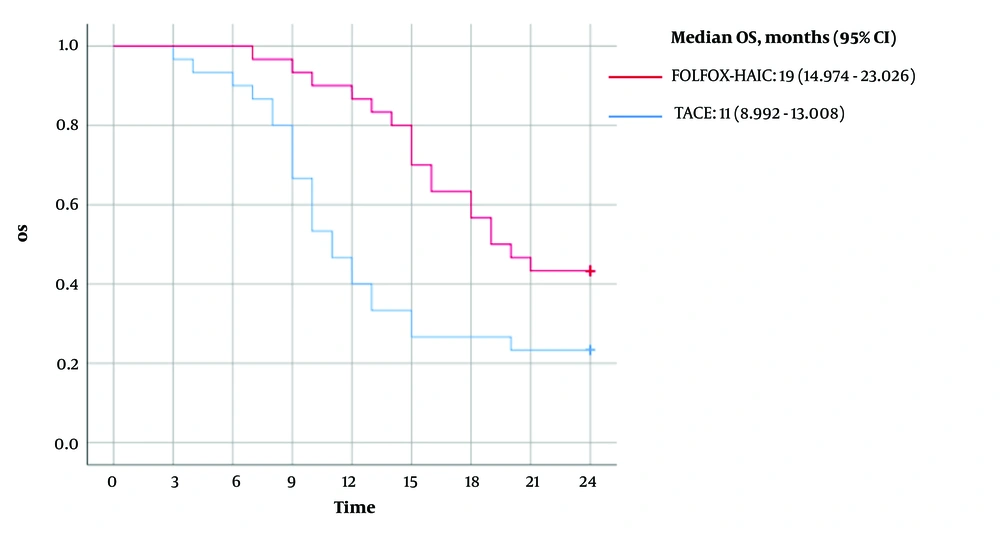
The mean OS for patients in the FOLFOX-HAIC group was 19 months (95% CI, 17.081 - 20.852), with a median OS of 19 months (95% CI, 14.974 - 23.026). In contrast, the mean OS for patients in the TACE group was 13 months (95% CI, 11.022 - 15.778), with a median OS of 11 months (95% CI, 8.992 - 13.008). A log-rank comparison between the two groups yielded a P-value of 0.007 (Figure 1).
4.3. Comparison of Progression-Free Survival Between Hepatic Arterial Infusion Chemotherapy Group and Transarterial Chemoembolization Group
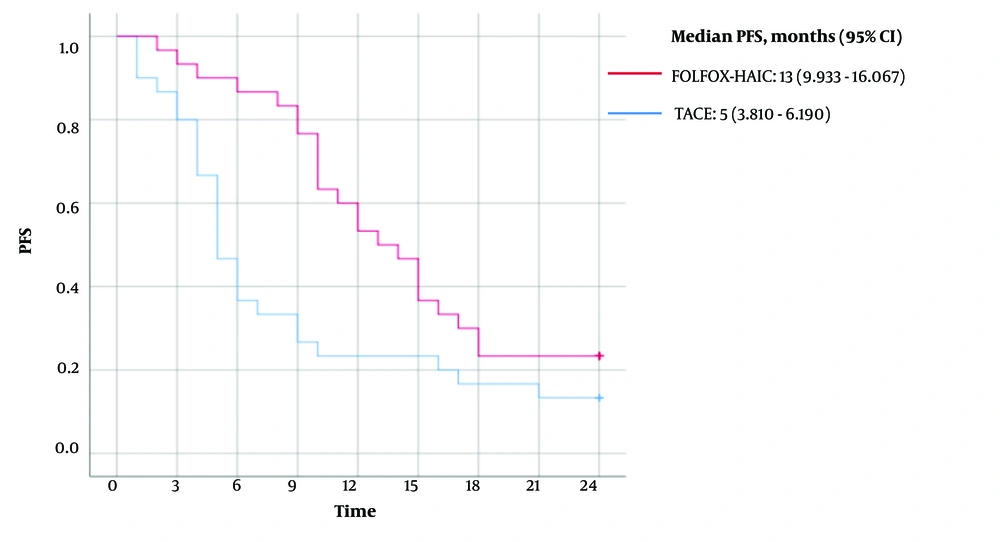
The mean PFS for patients in the FOLFOX-HAIC group was 14 months (95% CI, 11.771 - 16.562), with a median PFS of 13 months (95% CI, 9.933 - 16.067). In contrast, the mean PFS for patients in the TACE group was 9 months (95% CI, 5.977 - 11.356), with a median PFS of 5 months (95% CI, 3.810 - 6.190). A log-rank comparison between the two groups yielded a P-value of 0.016 (Figure 2).
4.4. Comparison of Symptomatic Progression-Free Survival Between Hepatic Arterial Infusion Chemotherapy Group and Transarterial Chemoembolization Group
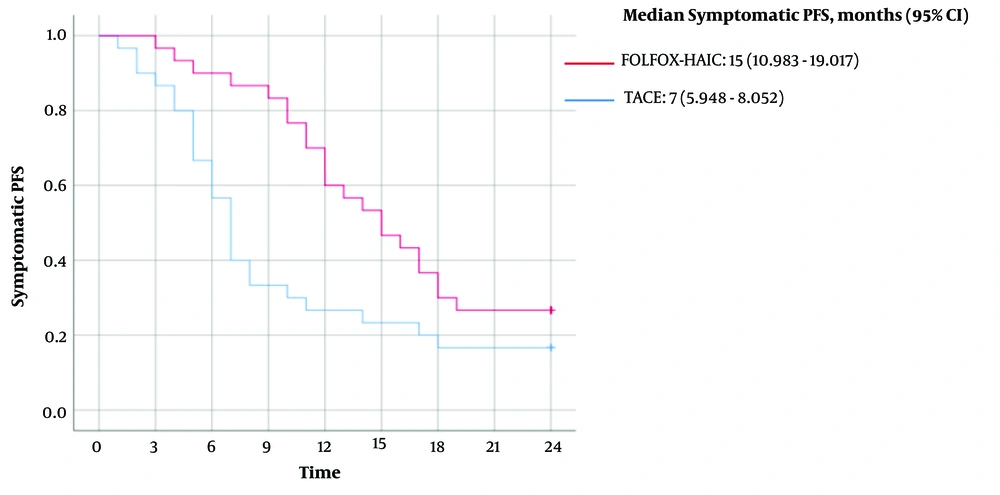
The mean symptomatic PFS for patients in the FOLFOX-HAIC group was 15 months (95% CI, 13.008 - 17.659), with a median symptomatic PFS of 15 months (95% CI, 10.983 - 19.017). In contrast, the mean symptomatic PFS for patients in the TACE group was 10 months (95% CI, 7.187 - 12.479), with a median symptomatic PFS of 7 months (95% CI, 5.948 - 8.052). A log-rank comparison between the two groups yielded a P-value of 0.019 (Figure 3).
4.5. Comparison of Recurrence Rate Between Hepatic Arterial Infusion Chemotherapy Group and Transarterial Chemoembolization Group
After a 2-year follow-up, the results showed that among the 30 patients who underwent primary liver cancer resection in the TACE group, 20 experienced postoperative recurrence, with a recurrence rate of 66.67%. In contrast, in the FOLFOX-HAIC group, out of the 30 patients who underwent primary liver cancer resection, 12 experienced postoperative recurrence, with a recurrence rate of 40.00%. Recurrence rates differed significantly between the two groups (χ2 = 4.286, P = 0.038).
4.6. Comparison of Adverse Events Between Hepatic Arterial Infusion Chemotherapy Group and Transarterial Chemoembolization Group
The study results showed that the main postoperative adverse reactions in the TACE group were embolization syndrome, including fever, gastrointestinal reactions, and abdominal pain. Twenty-one patients experienced adverse reactions during treatment, with an incidence rate of 70.00%. The FOLFOX-HAIC group had relatively fewer adverse reactions, mainly chemotherapy-related toxicities, which were generally mild. Twelve patients experienced adverse reactions during treatment, with an incidence rate of 40.00%. There was a significant difference in the incidence of AEs between the groups (χ2 = 5.455; P = 0.020).
5. Discussion
Liver cancer, a prevalent malignancy globally, presents a persistent challenge for the medical community due to its high incidence and mortality rates (25). Despite considerable progress in surgical techniques and an increase in liver cancer resection rates, postoperative recurrence remains a crucial factor affecting long-term survival in patients (26). Consequently, researching effective preventive interventional therapies to lower the recurrence risk post-liver cancer resection has emerged as a focal point of current studies. This study evaluated the efficacy of FOLFOX-HAIC and TACE in prophylactic interventional treatment after primary liver cancer resection, revealing significant differences between the two treatment modalities in terms of survival benefits, recurrence control, and safety.
The study results demonstrated that the FOLFOX-HAIC group was superior to the TACE group in key indicators such as OS, PFS, and symptomatic PFS, with a significantly reduced risk of recurrence and more favorable incidence and severity of AEs. Specifically, the FOLFOX-HAIC group exhibited significantly longer OS (P = 0.007), PFS (P = 0.016), and symptomatic PFS (P = 0.019) compared to the TACE group. Additionally, the recurrence rate was significantly lower in the FOLFOX-HAIC group (40.00%) than in the TACE group (66.67%, P = 0.038). In terms of adverse reactions, the TACE group primarily experienced embolization syndrome, with an incidence rate of 70.00% (21/30), whereas the FOLFOX-HAIC group mainly exhibited milder chemotherapy-related toxicity, with an incidence rate of 40.00% (12/30), and the difference was statistically significant (χ2 = 5.455; P = 0.020).
This finding not only provides important evidence-based support for the selection of adjuvant treatment strategies after primary liver cancer surgery but also triggers deeper considerations regarding the regulation of the liver cancer microenvironment, optimization of drug delivery systems, and precision treatment strategies. The survival advantage of FOLFOX-HAIC over conventional TACE may be rooted in its unique drug action mode and tumor biological effects. Firstly, from a pharmacokinetic perspective, HAIC creates a "drug pool" with high concentrations and prolonged exposure of chemotherapeutic agents locally in the tumor through continuous intra-arterial infusion (typically maintained for 24 - 48 hours) (27). The FOLFOX-HAIC not only overcomes the issue of uneven drug distribution caused by interrupted blood flow after embolization in TACE but also exerts a superimposed killing effect on tumor cells in different cell cycles by continuously inhibiting DNA synthesis (fluorouracil) and inducing DNA cross-linking (oxaliplatin) (28). Furthermore, the addition of leucovorin enhances the irreversible binding of fluorouracil to thymidylate synthase, further improving chemotherapy sensitivity (29). This synergistic effect targeting multiple pathways and mechanisms may effectively eliminate postoperative residual micrometastases and delay the recurrence process.
Secondly, TACE-induced embolization may trigger HIF-1α/VEGF-mediated angiogenesis and metastasis, whereas FOLFOX-HAIC avoids hypoxic stress and exerts anti-angiogenic effects through VEGF inhibition, potentially explaining its superior PFS outcomes (30-32). The onset and progression of primary liver cancer involve intricate, multifactorial, and multistep processes involving mutations and abnormal expressions of multiple genes, as well as disruptions in cellular signaling pathways (33). The carcinogenic process of hepatocytes is influenced by various internal and external factors, such as chronic hepatitis virus infections, exposure to aflatoxin, long-term alcohol consumption, and metabolic syndrome, which lead to genomic instability in hepatocytes and subsequently trigger malignant transformation of cells (34-36). During the development of liver cancer, the proliferation, invasion, and metastatic capabilities of tumor cells continuously enhance, and the special anatomical structure of the liver and its abundant blood supply provide favorable conditions for tumor recurrence and metastasis (37). Postoperative recurrence is a significant challenge in the treatment of primary liver cancer, with mechanisms mainly including the presence of micrometastases, enhanced invasiveness and angiogenic capacity of tumor cells, and suppression of the body’s immune function (38). Although surgical resection can eliminate macroscopically visible tumor lesions, micrometastases or residual cancer cells that may have existed preoperatively can rapidly proliferate postoperatively, leading to tumor recurrence (39). Meanwhile, surgical trauma and the reduction of tumor burden may temporarily suppress the body’s immune function, further promoting the recurrence and metastasis of tumor cells (40).
The study findings suggest that the recurrence rate was lower in the FOLFOX-HAIC group compared to the TACE group (40.00% vs. 66.67%), aligning with recent research highlighting the potential of HAIC in adjuvant liver cancer treatment. For example, He et al. found that HAIC combined with postoperative adjuvant therapy can reduce the risk of early recurrence, possibly through effective control of circulating tumor cells (CTCs) and MVI (41). It is noteworthy that postoperative recurrence of primary liver cancer often originates from pre-existing micrometastases or tumor cell dissemination caused by surgical manipulation, and traditional TACE may not cover all high-risk areas due to the deposition of embolic agents, especially around intrahepatic satellite lesions or portal vein tumor thrombi. In contrast, FOLFOX-HAIC can more thoroughly eliminate occult lesions through extensive infiltration of the liver parenchyma with high-concentration chemotherapeutic agents (42). Recent studies indicate that chemotherapeutic agents like oxaliplatin and fluorouracil may inhibit tumor recurrence by modulating the immune microenvironment — oxaliplatin induces immunogenic cell death and activates dendritic cells, while fluorouracil targets myeloid-derived suppressor cells (43). However, TACE-induced local inflammation may exacerbate immunosuppression, promoting a pro-recurrence microenvironment (44).
Adverse reactions are one of the important factors affecting patient tolerance and quality of life. In this study, 70% of patients in the TACE group experienced embolization syndrome (abdominal pain, fever, transient deterioration of liver function), while the FOLFOX-HAIC group mainly experienced mild chemotherapy-related toxicity (40%), with no reports of severe liver injury. This difference reflects the characteristics of the two techniques: Transarterial chemoembolization embolizes the tumor-feeding arteries with gelatin sponges or drug-eluting beads, inevitably causing ischemic injury to normal liver tissue, which may accelerate hepatic decompensation, especially in the context of cirrhosis; whereas FOLFOX-HAIC employs low-dose continuous infusion, resulting in lower systemic exposure of the drug after hepatic metabolism, thereby reducing classic chemotherapy toxicities such as myelosuppression (45). It is noteworthy that the toxicity profile of FOLFOX-HAIC is closely related to the characteristics of its drug combination. Dose-limiting toxicities of oxaliplatin, such as neurotoxicity, were not significantly observed in this study, possibly due to the short duration of adjuvant therapy and the low cumulative dose. Future studies need to further explore the optimal infusion duration and drug concentration to balance efficacy and safety.
Although FOLFOX-HAIC demonstrated comprehensive advantages in this study, treatment choices in clinical practice still need to be based on individual patient characteristics. For example, for high-risk patients with main portal vein tumor thrombi or extensive MVI, FOLFOX-HAIC may have greater advantages due to its potent penetration of vascular invasive lesions; whereas patients with low tumor burden and poor hepatic functional reserve may benefit from the local control and lower systemic toxicity of TACE (46). Additionally, precision therapy guided by molecular subtyping is on the rise: For instance, CTNNB1-mutant primary liver cancer responds poorly to traditional chemotherapy, while TP53-mutant types may be sensitive to oxaliplatin. The detection of such biomarkers is expected to further enhance the precision of adjuvant therapy (47, 48). It is also important to acknowledge that TACE may still be a preferable option in certain scenarios. For patients with compromised liver function, the lower systemic toxicity associated with TACE might be more advantageous, as it avoids the potential hepatotoxicity of continuous chemotherapy infusion. In cases of specific tumor subtypes that are less responsive to systemic chemotherapy, TACE could provide more effective local control. Furthermore, for patients who are intolerant to chemotherapy due to comorbidities or other reasons, TACE might be a more suitable alternative. The decision-making process should thus be tailored to the individual patient’s clinical condition, tumor characteristics, and overall treatment goals.
5.1. Conclusions
This study demonstrates that FOLFOX-HAIC, as a prophylactic interventional therapy after resection of primary liver cancer, is significantly superior to traditional TACE in prolonging survival, controlling recurrence, and improving safety. This finding not only provides high-level evidence for clinical practice but also suggests that the paradigm of adjuvant therapy for liver cancer is shifting from "local embolization-dominant" to "continuous infusion + systemic control". In the future, with the deep integration of molecular subtype and individualized treatment, FOLFOX-HAIC has the potential to become a standard adjuvant regimen for high-risk recurrent patients, ultimately enhancing the overall outcome for liver cancer patients.
5.2. Limitations of the Study
Despite our efforts to minimize bias and confounding, we acknowledge that this study has inherent limitations due to its retrospective nature. Firstly, the sample size of this study was relatively small (n = 60), which limited the ability to conduct subgroup analyses on potential confounding factors. Subgroup analyses could have further explored the impact of different patient characteristics on treatment outcomes. However, due to the limitation of the sample size, this study was unable to perform such analyses. Secondly, the short follow-up period (2 years) prevents the assessment of long-term recurrence rates beyond 5 years and the risks of secondary tumors. Radiomic or liquid biopsy indicators were not included, making it difficult to deeply analyze the predictors of efficacy differences. Future research should focus on the following directions: Conduct multicenter, large-sample phase III randomized controlled trials to validate the survival benefits of FOLFOX-HAIC; combine dynamic monitoring of circulating tumor DNA (ctDNA) to explore the association between molecular residual disease (MRD) clearance and prognosis; develop novel drug delivery systems, such as nanoparticles or immunomodulators combined with HAIC, to achieve temporal and spatial synergism between chemotherapy and immunotherapy.