1. Background
Liver cancer ranks among the most aggressive types of cancer, with approximately 905,000 new diagnoses and 830,000 fatalities reported globally in 2020 (1, 2). This positions it as the seventh most frequently occurring and the second most fatal cancer worldwide. The predominant subtype, hepatocellular carcinoma (HCC), accounts for 70% to 85% of all liver cancer cases, underscoring its significant impact on public health (3). Major risk factors for HCC include hepatitis B virus (HBV), hepatitis C virus (HCV), diabetes, obesity, and alcohol consumption, leading to higher rates in less developed regions (3, 4). Hepatic tumor cells have developed highly effective resistance mechanisms to chemotherapy, limiting the effectiveness of this widely used treatment for HCC (5, 6). Consequently, the three main surgical methods — liver transplantation, liver resection, and liver ablation — are regarded as the most effective treatments for HCC. However, none of these methods provide a definitive cure, and each has its own set of limitations (7). Despite the limited use of chemotherapy, some agents, including etoposide (ETO), have demonstrated significant efficacy in treating HCC (5, 8). Etoposide, an anticancer drug derived from plants, inhibits cancer cell growth and induces cell death by interacting with DNA and the Topoisomerase II (Top II) enzyme. This action prevents the rejoining of DNA double strands, thereby disrupting the cell’s ability to replicate and repair its genetic material (9, 10). Due to the various side effects associated with ETO in patients, exploring innovative approaches, such as combination therapy, is considered a method to reduce these negative effects while also enhancing its anticancer effectiveness (11, 12).
The p53 protein, a vital tumor suppressor, plays a central role in controlling the cell cycle, facilitating DNA repair, and inducing apoptosis when DNA damage is irreparable (13, 14). Mutations in the p53 gene are present in approximately 60% of all cancers, including HCC. These mutations can result in either a gain or loss of function, both of which are key factors in tumor development (15). Additionally, p53 gene mutations have been shown to increase the chemoresistance of liver cancer cells, making them less responsive to treatment (2, 16). Therefore, monitoring the status of the p53 gene is essential in cancer research and treatment strategies.
Exosomes, tiny vesicles secreted by various cell types, have attracted considerable interest due to their potential in addressing diseases such as cardiovascular conditions, neurodegenerative diseases, and cancer (17, 18). Wharton’s jelly-derived mesenchymal stem cell (WJ-MSC) exosomes possess a unique molecular profile rich in anti-inflammatory and pro-apoptotic factors. They are characterized by low immunogenicity, making them safe for therapeutic use with minimal risk of immune rejection. Additionally, their enhanced homing ability to tumor microenvironments increases their effectiveness in targeted cancer therapy (19, 20). These vesicles are emerging as powerful agents in cancer therapy because of their role in cell communication and modulation of the tumor microenvironment. Exosomes can induce apoptosis and inhibit the proliferation and migration of various cancer cell lines by influencing key signaling pathways, including Erk1/2, PI3K, and NF-κB (21, 22). They are thought to exert their anticancer effects by utilizing antioxidant properties, anti-inflammatory actions, and the delivery of bioactive molecules (23). Despite their promise, the effects of exosomes combined with ETO on HCC cell lines remain to be explored.
2. Objectives
The present study seeks to explore how the combination of exosomes derived from stem cells and ETO influences apoptosis and the regulation of p53 protein expression in HCC cell lines.
3. Methods
3.1. Cell Culturing and Reagent Preparation
Hep-G2 cells were obtained from the Pasteur Institute (Tehran, Iran) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The medium was replaced 24 hours before experiments with a serum-free version. Cells were grown at 37°C in a 5% CO2 incubator. The exosome suspension was then diluted in DMEM to the required concentration for the experiments.
3.2. Isolation and Characterization of Wharton’s Jelly-Derived Mesenchymal Stem Cells
Umbilical cords were obtained from full-term cesarean deliveries and carefully processed to extract Wharton’s Jelly. This tissue was cultured in DMEM supplemented with 10% FBS at 37°C for cell growth. The cells were subcultured between passages 3 - 5 for further analysis. Differentiation capabilities were evaluated by inducing osteogenic and adipogenic lineages, and the cells were stained with Alizarin Red and Oil Red O to confirm their respective potentials. For surface marker identification, flow cytometry was employed using antibodies against CD44, CD105, CD45, and CD34. Flow cytometry analysis was performed on a BD FACSLyric machine, with a minimum of 20,000 events recorded per sample.
3.3. Exosome Isolation and Analysis
Exosomes were obtained from the culture medium of WJ-MSCs grown in DMEM with 15% FBS, which was then replaced with serum-free media before the isolation process. After centrifugation to eliminate cell debris, exosomes were extracted using a commercial extraction kit following the manufacturer’s protocol. The exosome suspension was stored at -80°C for further use. The protein concentration of the isolated exosomes was determined through a BCA protein assay. The particle size distribution was evaluated using dynamic light scattering (DLS) with a Nano-Zetasizer, and the exosome morphology was examined under a transmission electron microscopy (TEM) after staining with uranyl acetate.
3.4. Real-time PCR for Gene Expression Analysis
RNA was extracted from 1×106 HepG2 cells for cDNA synthesis, following the protocol provided by the manufacturer. After reverse transcription, quantitative real-time PCR was performed to assess the expression levels of the apoptotic genes Bax and Bcl-2, along with the housekeeping gene GAPDH. The reactions were conducted in 20 µL volumes using a real-time PCR system, and the primer sequences for each gene are shown in Table 1. GAPDH was used as an internal control to normalize the results.
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| Bax | CATGGGCTGGACATTGGACT | CGTGGGCGTCCCAAAGTA |
| Bcl-2 | GCCGTGTTACTTGTAGTGTGT | TTCCACAAAGGCATCCCAGC |
| GAPDH | CCATGGGGAAGGTGAAGGTC | GCAGGAGGCATTGCTGATGA |
3.5. Western Blot Analysis
Total proteins from cell lysates were extracted using a RIPA buffer and separated by SDS-PAGE. The proteins were transferred to PVDF membranes and blocked with 5% milk in TBST. Membranes were incubated overnight with primary p53 antibodies at 4°C, followed by HRP-conjugated secondary antibodies. Protein bands were detected using an ECL system, and protein levels were quantified using ImageJ. β-actin was used as the loading control for normalization.
3.6. Flow Cytometry
Apoptosis was evaluated using an Annexin V-FITC/PI apoptosis detection kit. After treatment, cells were collected by centrifugation and resuspended in calcium-based binding buffer with Annexin V-FITC. Following a 20-minute incubation at 4°C, propidium iodide (PI) was added, and the samples were incubated for an additional 10 minutes. Apoptotic cells were analyzed via flow cytometry, and the results were processed using FlowJo software.
3.7. Caspase Activity Measurement
Caspase-3 and caspase-9 activities were assessed using a colorimetric assay. Cell lysates from treated groups were incubated with specific caspase substrates at 37°C for 1 hour. The cleavage of substrates by active caspases caused a color shift, which was quantified by measuring absorbance at 405 nm using a microplate reader.
4. Results
4.1. Wharton’s Jelly-Derived Mesenchymal Stem Cells Characterization
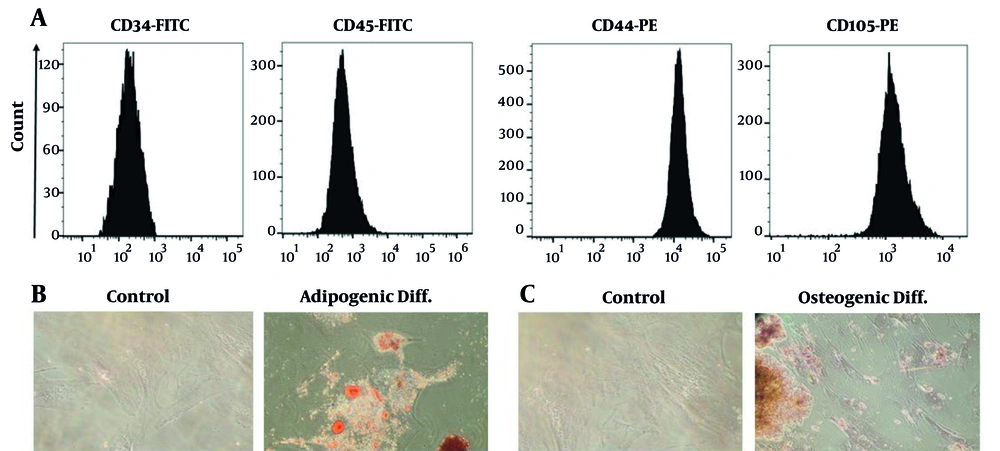
The WJ-MSCs were evaluated for their surface marker profile and ability to differentiate into various cell types. At passage 3, the cells formed fibroblast-like monolayers and adhered to the culture flasks. Flow cytometry analysis revealed positive expression of CD44 and CD105 markers, and the absence of CD34 and CD45 markers, confirming the isolation of MSCs (Figure 1A). Differentiation assays demonstrated adipogenic potential, with lipid droplet formation confirmed by Oil Red O staining, and osteogenic differentiation verified through Alizarin Red staining, showing typical calcium deposits (Figure 1B and C).
A, Flow cytometric analysis showed that the isolated Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) were strongly positive for CD44 and CD105, while lacking CD34 and CD45, confirming their mesenchymal identity; B, after 21 days of adipogenic induction, Oil Red O staining revealed numerous lipid droplets within the cells, indicating successful adipocyte differentiation; C, osteogenic differentiation was confirmed by Alizarin Red staining, which highlighted substantial calcium deposition in the induced cells after 21 days.
4.2. Exosome Characterization
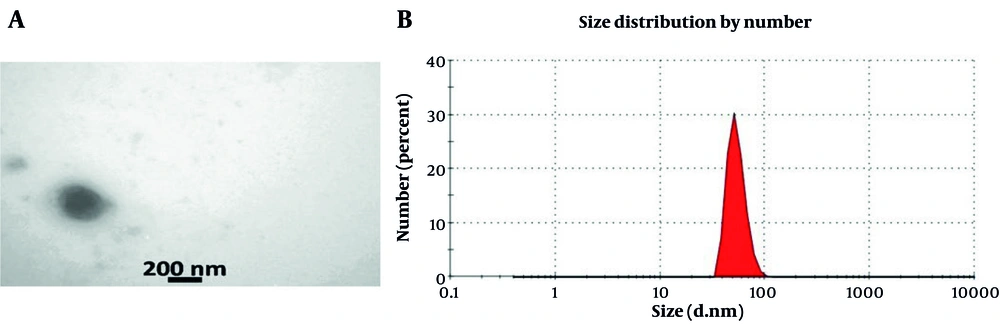
Transmission electron microscopy was employed to characterize the isolated exosomes, which revealed a spherical shape ranging from 50 to 200 nm in diameter (Figure 2A). Zeta sizer analysis showed that around 80% of the exosomes had a diameter of approximately 73 nm, confirming the expected size distribution and purity of the exosomes (Figure 2B).
A, Transmission electron microscopy (TEM) images showed that the isolated exosomes had a rounded and vesicular structure, typical of extracellular vesicles; B, size profiling with the Zeta Sizer demonstrated that nearly 85% of the exosomes had an average diameter close to 73 nm, indicating a narrow and consistent size distribution.
4.3. Synergistic Effect of Exosomes and Etoposide on HepG2 Cell Proliferation
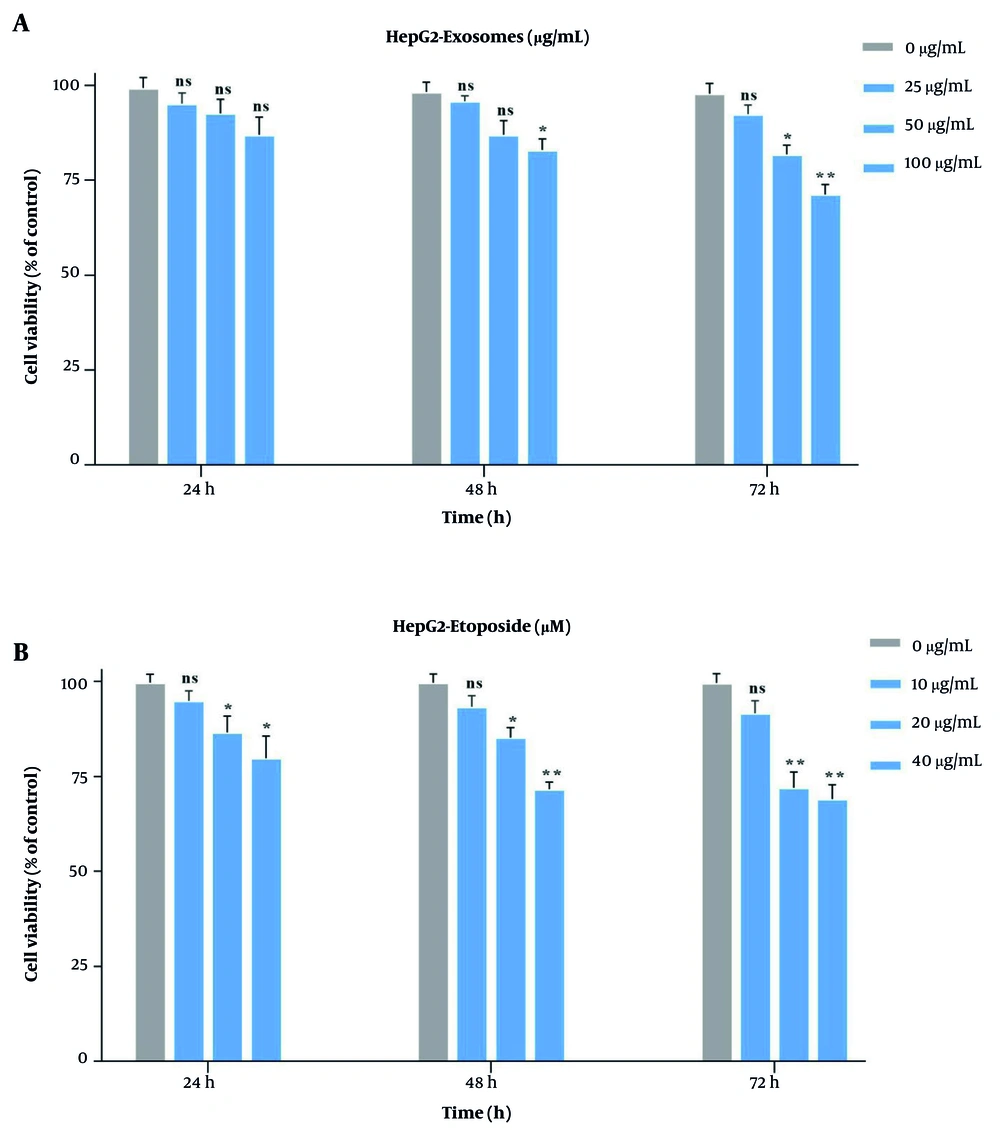
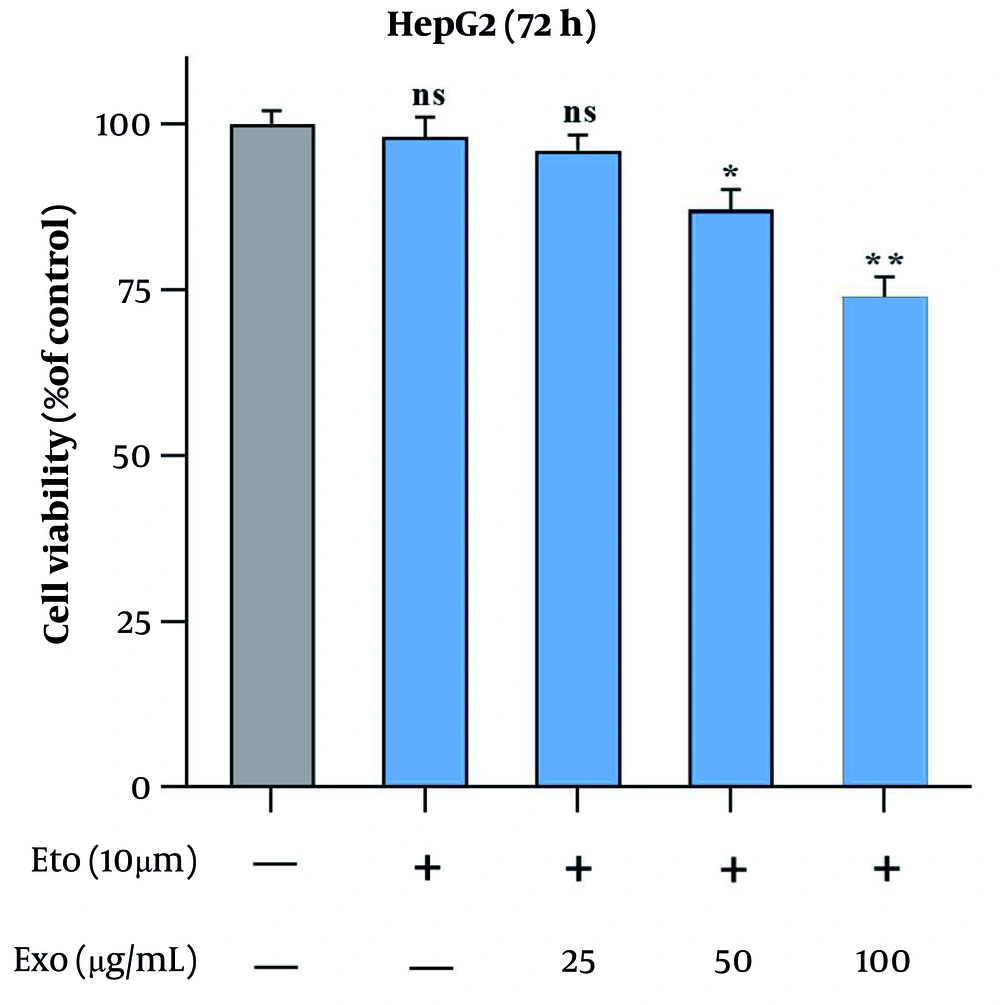
To assess the optimal doses, HepG2 cells were exposed to different concentrations of stem cell-derived exosomes (25, 50, 100 µg/mL) and ETO (10, 20, 40 µM) individually (Figure 3A and B). The MTT assays revealed that both agents reduced cell viability in a time- and dose-dependent manner. Exosomes at 100 µg/mL showed the highest inhibitory effect after 72 hours (~ 33%), while 10 µM ETO alone had minimal impact across all time points. However, 20 and 40 µM ETO significantly suppressed cell growth. None of the single treatments reached IC50. When 10 µM ETO was combined with increasing doses of exosomes, cell growth was more effectively suppressed compared to ETO alone. The combination of 100 µg/mL exosomes and 10 µM ETO yielded the strongest inhibitory effect (Figure 4). Combination Index Analysis confirmed a synergistic interaction, with the most potent synergy (CI = 0.36) observed in this group (Table 2), which was used for subsequent experiments.
A, The cytotoxic effects of stem cell-derived exosomes on HepG2 cells were assessed using MTT assay at 24-, 48-, and 72-hours post-treatment with increasing concentrations; B, similarly, etoposide (ETO) was applied at ascending concentrations for the same time intervals to evaluate its inhibitory impact on cell viability. (* P < 0.05, ** P < 0.01; ns, not significant).
The effects of stem cell-derived exosomes combined with etoposide (ETO) on HepG2 cell proliferation were evaluated using the MTT assay. Cells were treated with 10 µM ETO alongside 25, 50, and 100 µg/mL of exosomes for 72 hours. (* P < 0.05, ** P < 0.01; ns, not significant compared to control).
| Variable | Exo | ||
|---|---|---|---|
| 25 µg/mL | 50 µg/mL | 100 µg/mL | |
| ETO (10 μm) | 0.96 | 0.72 | 0.36 |
Abbreviation: ETO, etoposide.
a Hep-G2 cells incubated with 10μM of etoposide and various concentrations of exosomes. CI was calculated by CompuSyn software. CI < 1.0 represents synergism, CI = 1.0 represents an additive, and CI > 1.0 represents antagonism.
4.4. Exosomes Enhance Etoposide-Induced Apoptosis in HepG2 Cells
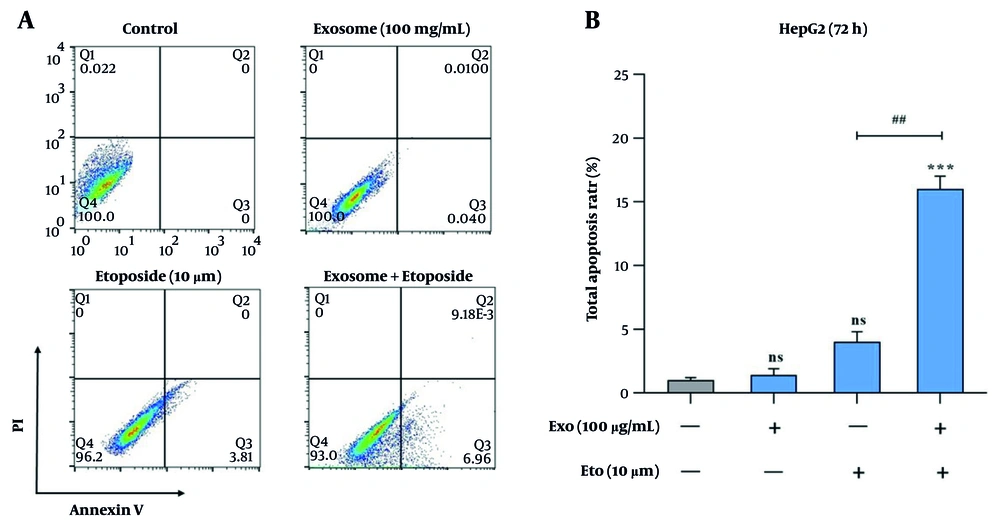
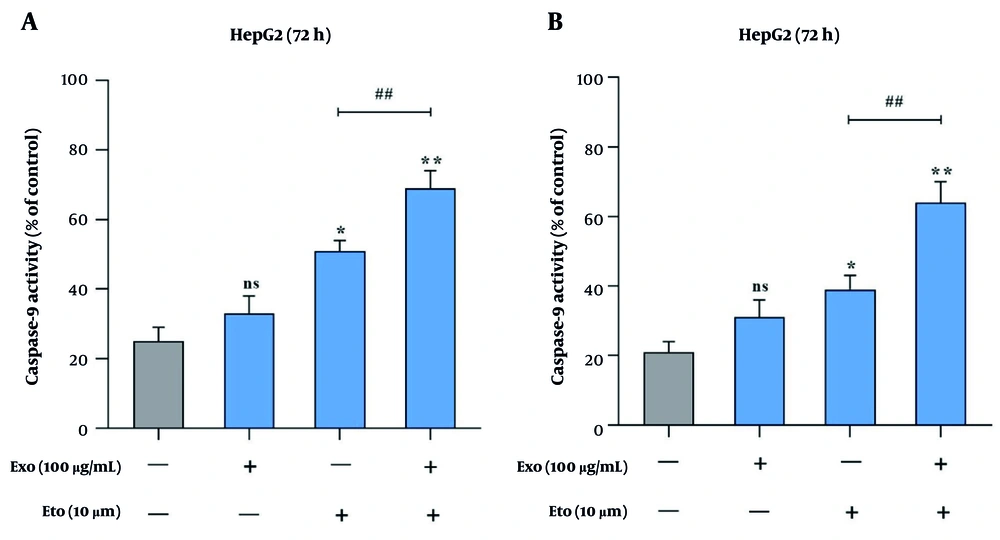
Apoptosis induction was assessed using Annexin V/PI staining and caspase activity assays. Treatment with exosomes or ETO alone did not result in a significant increase in apoptotic cell death compared to the control group. Co-treatment with exosomes and ETO elevated apoptosis significantly to 16.2%, indicating a synergistic effect (Figure 5A and B). Caspase-9 activity was 33% with exosomes and 51% with ETO alone, while their combination raised it to 69% (Figure 6A). Similarly, caspase-3 activity increased from 31% (exosomes) and 39% (ETO) to 64% under combined treatment (Figure 6B), further supporting the potentiating role of exosomes in ETO-mediated apoptosis.
Evaluation of apoptosis induction in HepG2 cells following treatment with exosomes and etoposide (ETO). A, Representative flow cytometry dot plots showing apoptotic cell populations after treatment with exosomes, ETO, or their combination; B, quantitative analysis of apoptotic cells presented as bar graphs. (*** P < 0.001 vs. control; ## P < 0.01 vs. ETO alone).
Caspase activity analysis in HepG2 cells following treatment with exosomes and etoposide (ETO). A, Caspase-9 activity levels in response to individual and combined treatments; B, caspase-3 activity levels under the same conditions. (* P < 0.05, ** P < 0.01 vs. control; ## P < 0.01, vs. ETO alone).
4.5. Exosomes and Etoposide Alter Bax/Bcl-2 Expression
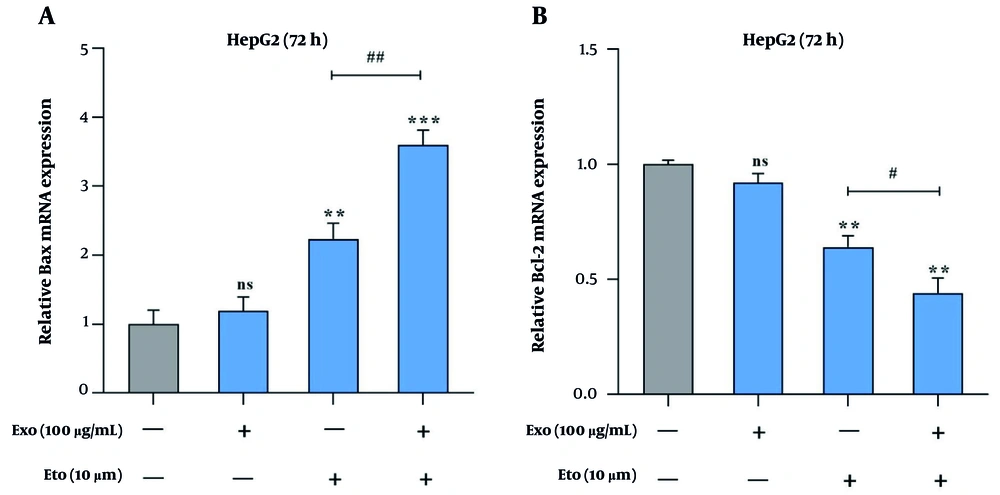
The qRT-PCR analysis revealed that the combination of exosomes and ETO significantly shifts the Bax/Bcl-2 expression ratio toward apoptosis. Individually, exosomes and ETO elevated Bax expression by 1.2- and 2.3-fold, while co-treatment amplified it to 3.6-fold (Figure 7A). Conversely, Bcl-2 expression decreased to 0.92- and 0.64-fold with single treatments, and further dropped to 0.44-fold with the combination (Figure 7B). This enhanced Bax/Bcl-2 ratio reflects the activation of the intrinsic apoptotic pathway. The data suggest that exosomes sensitize HepG2 cells to ETO by modulating key regulators of mitochondrial-mediated apoptosis.
Effects of exosomes and etoposide (ETO) on the expression of apoptosis-associated genes in HepG2 cells. A, Relative expression of Bax in cells treated with exosomes, ETO, or their combination; B, relative expression of Bcl-2 under the same treatment conditions. (** P < 0.01, *** P < 0.001 vs. control; # P < 0.05, ## P < 0.01 vs. ETO alone).
4.6. Exosomes and Etoposide Synergistically Enhance p53 Expression
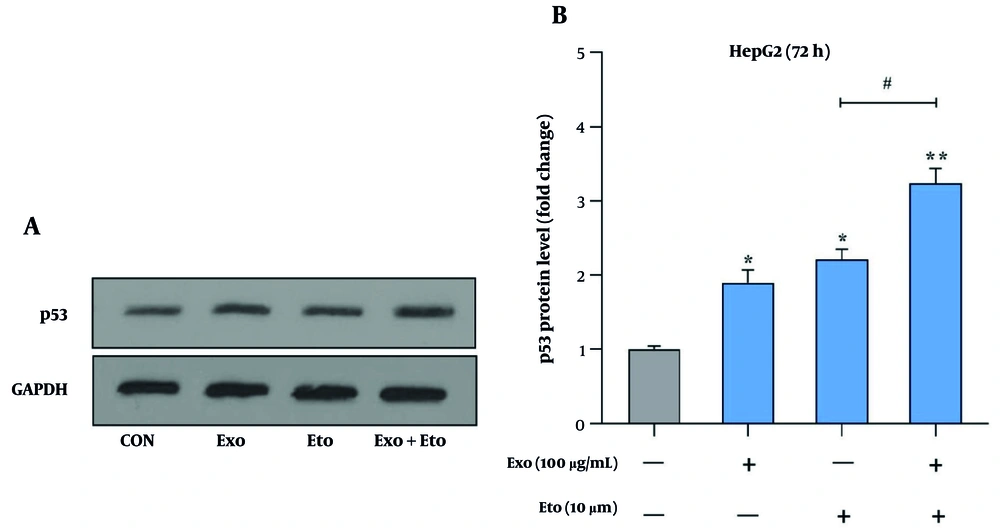
We further evaluated the impact of exosomes and ETO on apoptosis by analyzing p53 protein levels. Both exosomes and ETO individually caused a significant increase in p53 expression, with 1.9- and 2.2-fold increases, respectively. However, the combination of exosomes and ETO resulted in a more substantial increase, with p53 levels rising to 3.24-fold (Figure 8A and B). This suggests that exosomes enhance the ability of ETO to upregulate p53, a critical tumor suppressor involved in cell cycle regulation and apoptosis, thus potentiating the overall apoptotic response in HepG2 cells.
Analysis of p53 protein expression in HepG2 cells following treatment with exosomes and etoposide (ETO). A, Western blot image showing the levels of p53 protein in response to treatments with exosomes, ETO, or their combination. GAPDH was used as a loading control (~36 kDa); B, quantitative analysis of p53 band intensity normalized to GAPDH. (* P < 0.05, ** P < 0.01 vs. control; # P < 0.05 vs. ETO alone).
5. Discussion
Cancer remains one of the most challenging diseases, and researchers continue to explore effective therapies. Among these, HCC stands out due to its aggressive progression, high mortality rate, and resistance to standard treatments such as chemotherapy and radiation therapy (24-26). These therapies often become ineffective as tumors develop resistance, leading to the need for higher drug doses, which consequently increase side effects (27, 28). To address this challenge, there is a growing focus on alternative treatments that are less toxic but equally effective, such as therapies involving stem cell-derived exosomes (29). Exosomes, small vesicles secreted by stem cells, have emerged as promising vehicles for drug delivery and cellular communication, showing potential in enhancing cancer therapies with minimal adverse effects (30).
Apoptosis, the programmed cell death mechanism, plays a critical role in maintaining cellular homeostasis and protecting the organism from malignant transformation (31). Cancer cells often evade apoptosis through various mechanisms, including mutations in pro-apoptotic and anti-apoptotic proteins like Bax and Bcl-2, respectively (32). These mutations enable cancer cells to survive longer than normal cells, proliferate uncontrollably, and resist chemotherapeutic treatments (33, 34). Various signaling pathways, such as p53, NF-kB, and caspases, regulate apoptosis. The dysregulation of these pathways often leads to the failure of apoptosis, providing cancer cells with a survival advantage (35).
Exosomes, with their unique properties, have garnered significant interest in cancer treatment. Studies have shown that exosomes can modulate various signaling pathways, enhance the effects of chemotherapy, and directly contribute to apoptosis (36). In recent years, exosome-based therapies have been explored in combination with conventional chemotherapy to overcome the limitations of single-agent treatments (37). By facilitating the delivery of therapeutic molecules and enhancing immune responses, exosomes have shown potential in amplifying the effects of chemotherapeutic agents, thus promoting cancer cell apoptosis and inhibiting tumor growth (38).
In this study, we examined the synergistic effects of exosomes and ETO in HepG2 cells, a human liver cancer cell line. The results revealed that both exosomes and ETO, when used individually, significantly reduced cell viability in a dose- and time-dependent manner. However, when combined, the two agents exhibited a synergistic effect, enhancing cell death and inhibiting cancer cell growth more effectively than either treatment alone. This observation is in line with prior research suggesting that exosomes can enhance the therapeutic efficacy of chemotherapeutic agents such as ETO by regulating apoptotic signaling pathways, as reported by Vahabi et al. (39).
We also observed significant changes in the expression of key apoptotic genes following combined treatment. The combination of exosomes and ETO led to an increased expression of Bax, a pro-apoptotic protein, and a decrease in the expression of Bcl-2, an anti-apoptotic protein, suggesting that exosomes enhance the pro-apoptotic effects of ETO. These results were further corroborated by increased caspase-3 and caspase-9 activities, which are central to the execution of apoptosis. The modulation of Bax and Bcl-2 expression, together with the activation of caspases, indicates that the combined treatment induces apoptosis through the mitochondrial pathway, a crucial pathway for apoptosis induction in cancer cells (40).
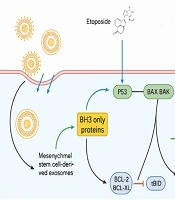
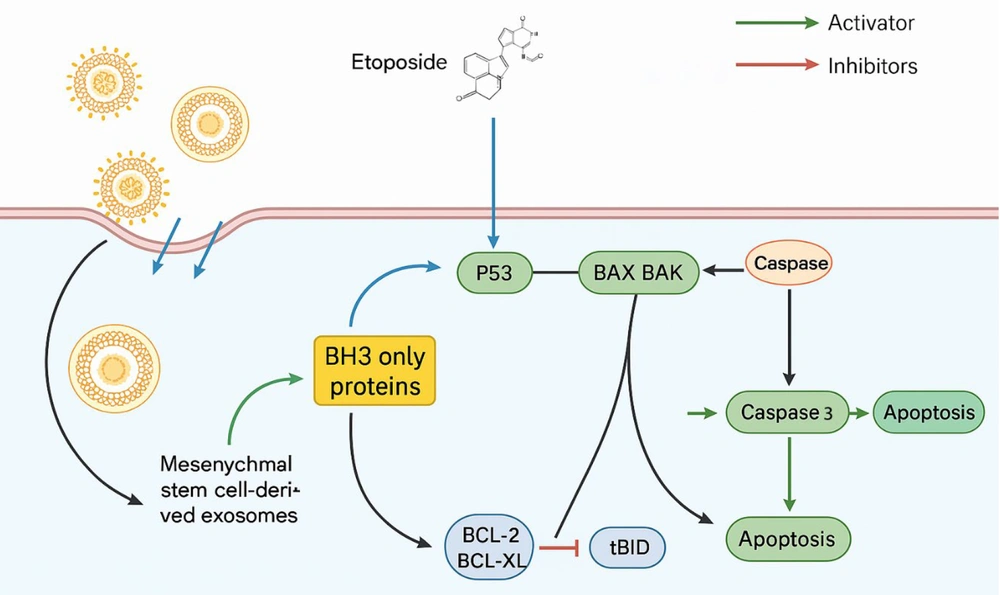
In addition to gene expression changes, we investigated the role of p53, a key tumor suppressor involved in regulating apoptosis (41). Our results showed that the combined treatment of exosomes and ETO significantly upregulated p53 protein levels, further confirming the synergistic effects of these agents. p53 plays a critical role in regulating the cell cycle and promoting apoptosis in response to cellular stress or DNA damage (Figure 9). By enhancing p53 expression, exosomes may help restore the apoptotic potential of cells that have compromised p53 activity due to mutations or other alterations commonly observed in cancer cells.
Schematic representation of apoptosis induction in HepG2 cells treated with exosomes and etoposide (ETO). The combined treatment elevates p53 protein levels and upregulates pro-apoptotic Bax expression while downregulating anti-apoptotic Bcl-2. These molecular changes activate caspase pathways, particularly caspase-9 and caspase-3, leading to enhanced apoptosis in liver cancer cells.
The current study utilized a monoculture model of HepG2 cells, which does not fully replicate the complexity of the HCC tumor microenvironment. This environment includes interactions with hepatic stellate cells, immune cells, and endothelial cells, as well as inflammation and fibrosis processes. Future studies should incorporate co-culture systems or 3D models to better mimic these conditions and validate the therapeutic effects observed.
5.1. Conclusions
In conclusion, this study demonstrates that stem cell-derived exosomes enhance the therapeutic effects of ETO in HepG2 liver cancer cells by promoting apoptosis through modulation of apoptotic genes and activation of related pathways. While these promising in vitro findings suggest potential improvements in chemotherapy outcomes for liver cancer, further in vivo studies are essential to confirm the efficacy and safety of this combination before clinical application. Future research should focus on validating these results in relevant animal models and exploring advanced drug delivery systems to improve targeted delivery and therapeutic efficiency.