1. Background
Spontaneous bacterial peritonitis (SBP) is defined as an infection of ascetic fluid without a proven intra-abdominal source (1, 2). SBP is a common and severe complication in cirrhotic patients (3), and is the major cause of mortality and morbidity among them (4). Bacterial infection due to complications such as hepatic encephalopathy or hepatic failure, disruption of homeostasis, and renal insufficiency are common causes of the increased morbidity and mortality rates in cirrhotic patients (5, 6). One of the required measures for SBP patients is rapid diagnosis of peritonitis for empirical antibiotic treatment (7, 8). Diagnosis of SBP is based on the number of ascetic fluid cells as polymorphonuclear cell count (PMN) ≥ 250/mm3 in ascetic fluid. Also, only 50 to 70% of a patient’s positive cultures for ascetic fluid are generally reported (9-11). Due to limitations such as the time-consuming process for the diagnosis of peritonitis ascetic fluid and the lack of access to appropriate laboratory facilities, reports of negative cultures could be delayed (12, 13). Given these circumstances, numerous studies have been done on the available assessment techniques and rapid diagnosis of peritonitis.
It seems that some inflammatory cytokines can be used to determine the presence and severity of an inflammatory response and organ failure (14-16). The C-reactive protein is an acute phase protein that is produced predominantly by liver hepatocytes after stimulus by many cytokines and conditions such as infection or inflammation (17-20). The marker is one of the most common clinical and inflammatory indicators that can be measured in any laboratory (21, 22). A few studies have shown increased levels of this marker in cirrhotic patients (1, 2, 23). For instance, in the study by Preto-Zamperlini et al., increased serum levels of CRP in children with SBP were demonstrated (24).
2. Objectives
We investigated the accuracy, sensitivity, and specificity of CRP compared to the PMN count of cultured ascetic fluid for the diagnosis of SBP in children.
3. Methods
This prospective study was conducted as a single-center cross-sectional cohort study during July 2013 to August 2014 in the department of pediatric gastroenterology in Nemazee Teaching Hospital, which is affiliated with Shiraz University of Medical Sciences, the major referral center in southern Iran. This study was approved by the ethics committee of Shiraz University of Medical Sciences, and all parents were asked to provide informed consent. All patients aged two months to 18 years, regardless of gender, who were admitted for liver disease and ascites were candidates for the sample for our study, and underwent abdominal paracentesis procedures for clinical suspicion of SBP.
The exclusion criteria were as follows: 1, receiving antibiotics during the past week or outside the hospital for any reason; 2, history of abdominal surgery in the past month; 3, renal dysfunction; 4, secondary bacterial peritonitis; 5, peritonitis carcinomatosis; 6, pancreatic peritonitis; 7, malignancy, or 8, tuberculosis. Children with other sources of infection were also not included, along with children with evidence of nephritic syndrome or heart failure. Based on the exclusion and inclusion criteria, 150 children with liver disease were included. After selection, patient paracentesis of ascetic fluid was performed on all patients. Cell blood count was determined, and serum samples were also obtained. Samples were sent to a specialized laboratory and subjected to laboratory, cytology, and bacteriology culture evaluation. WBC counts (Sysmex, USA), erythrocyte sedimentation rate (ESR), CRP levels, prothrombin time (PT), international normalized ratio (INR), serum total protein levels, serum albumin levels (Sigma Aldrich, St. Louis, USA), Alanine transaminase (ALT) levels, and Aspartate transaminase (AST) levels (Sigma Aldrich, St. Louis, USA) were determined for each case.
Diagnosis of SBP was based on PMN cell count ≥ 250/mm3 in ascetic fluid (25). In this study, serum was obtained from patients within the first hours of admission for measurement of CRP. Quantitative CRP was measured by the immunoturbidimetry method (Biorex, Antrim, UK). The cut off point for CRP in the study was 6 mg/dL.
Data were expressed as means ± SD for quantitative variables, and as frequencies for qualitative variables. The normality was continuously assessed with a Kolmogorov-Smirnov test. For the CRP marker compared to the PMN count by means of a positive culture test, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated according to a standard formula. Also, the area under the curve (AUC) of the receiver operating characteristic (ROC) curves was applied to assess the predictive power of CRP. The data were assessed by simple linear regression analysis to determine the correlations between CRP values and other markers related to liver function, such as albumin, protein, PT, and bilirubin levels. A level of P < 0.05 was considered statistically significant. Statistical analysis of the data was performed using the SPSS 19) SPSS Inc., IL, Chicago, USA) software package.
This study was approved by the ethical committee of Shiraz University of Medical Sciences (No. EC-P-9363-6513).
4. Results
Basic characteristics of the patients are shown in Table 1. In total, information about 150 patients with cirrhosis of the liver was investigated; 72.7% of the patients presented without SBP, and 27.3% of patients were diagnosed with SBP. The most common symptoms in patients without SPB were abdominal pain (67.9%) and abdominal distension (66.05 %). In patients with SBP, the most common clinical symptoms were also abdominal pain (70.7%) and abdominal distension (58.5%).
| Basic Characteristic | Without SBP (n=109) | With SBP (n=41) | P Value |
|---|---|---|---|
| Age, y, mean ± SD | 5.02 ± 4.49 | 4.71 ± 4.45 | 0.646 |
| Sex, No. (%) | 0.881 | ||
| Male | 57 (52.30) | 22 (53.66) | |
| Female | 52 (47.70) | 19 (46.34) | |
| Symptoms, No. (%) | |||
| Vomiting | 31 (28.44) | 13 (31.70) | 0.689 |
| Abdominal pain | 74 (67.88) | 29 (70.73) | 0.738 |
| Abdominal distention | 72 (66.05) | 24 (58.53) | 0.393 |
| Irritability | 27 (24.77) | 8 (19.51) | 0.498 |
| Loss of consciousness | 14 (12.84) | 6 (14.63) | 0.774 |
| Fever | 61 (55.96) | 27 (65.85) | 0.237 |
| Poor feeding | 31 (28.44) | 8 (19.51) | 0.267 |
| Etiology of Liver Disease, No. (%) | 0.864 | ||
| Idiopathic Neonatal Hepatitis (INH) | 21 (19.26) | 10 (24.39) | |
| Congenital Hepatic Fibrosis (CHF) | 7 (6.42) | 0 | |
| Biliary Atresia | 32 (29.35) | 13 (31.70) | |
| Tyrosinemia | 9 (8.28) | 1 (2.43) | |
| Autoimmune Hepatitis (AIH) | 4 (3.66) | 0 | |
| Idiopathic Cirrhosis | 7 (6.42) | 1 (2.43) | |
| Fulminant Hepatitis | 5 (4.58) | 5 (12.19) | |
| Wilson’s disease | 10 (9.17) | 7 (17.07) | |
| Other | 14 (12.84) | 4 (9.75) |
The etiology of cirrhosis revealed that the most common causes of cirrhosis were biliary atresia (29.3%), neonatal hepatitis (19.26%), and Wilson’s disease (17.9%) in patients without SBP. In patients with SPB, the most common causes of cirrhosis were also biliary atresia (31.7%), neonatal hepatitis (24.4%), and Wilson’s disease (17.7%).
Among the 150 cases, 17 cases had positive cultures for the following: E. coli (n=7); Acinetobacter (n = 2); Klebsiella (n = 2); Streptococcus pneumoniae (n = 2); and Enterococcus (n = 3).
The different results of the laboratory findings of cirrhotic patients without SBP and those with SBP are shown in Table 2. Evaluation of ascites fluid showed that the mean ± SD of total cell counts in patients with SBP (1986.54 ± 234.54) was significantly higher compared to the counts for patients without SBP (725.46 ± 134.35)(P < 0.0001). The means ± SD of white blood cell (WBC) count and polymorph nuclear cell (PMN) count in patients with SBP were significantly higher than the counts for patients without SBP (P < 0.0001) (Table 2).
| Data | Without SBP (n = 109) | With SBP (n = 41) | P Value (Unpaired t-Test) |
|---|---|---|---|
| Ascetic fluid | |||
| WBC count, µL | 170.54 ± 87.65 | 1114.5 ± 234.3 | < 0.0001 |
| PMN count, µL | 46.56 ± 34.23 | 945.3 ± 342.4 | < 0.0001 |
| Protein, g/dL | 1.78 ± 1.17 | 2.6 ± 1.26 | < 0.0001 |
| Albumin, g/dL | 0.79 ± 0.43 | 1.3 ± 0.8 | < 0.0001 |
| Sugar | 81.43 ± 25.3 | 79.9 ± 23.7 | 0.654 |
| LDH, U/L | 160.65 ± 76.4 | 398.54 ± 87.6 | < 0.0001 |
| Serum | |||
| WBC, µL | 8029.4 ± 332.3 | 13587.6 ± 240.4 | 0.005 |
| PMN, µL | 1766.54 ± 435.32 | 3024.5 ± 265.4 | 0.345 |
| ALT, IU/L | 151.86 ± 34.3 | 177.2 ± 34.5 | 0.523 |
| AST, IU/L | 264.5 ± 65.4 | 186.5 ± 34.2 | 0.240 |
| Protein, g/dL | 4.9 ± 1.2 | 4.9 ± 2.3 | 0.859 |
| Albumin, g/dL | 3.07 ± 1.23 | 3.23 ± 1.3 | 0.310 |
| PT (s) | 17.29 ± 10.43 | 17.86 ± 23.2 | 0.738 |
| INR | 19.66 ± 12.54 | 14.06 ± 12.2 | 0.366 |
| Total bilirubin, µmol/L | 132.3 ± 23.2 | 120.6 ± 23.4 | 0.382 |
| ESR, mm/h | 20.14 ± 7.65 | 32.8 ± 23.2 | 0.002 |
| CRP, mg/L | 21.59 ± 15.43 | 36.9 ± 23.4 | 0.001 |
Abbreviations: ALP, Alkaline phosphates; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CRP, C-reactive protein; ESR, estimated sedimentation rate; INR: international normalization rate; LDH: lactate dehydrogenate; PT: prothrombin time; PMN: polymorphonuclear cell; WBC: White blood cell.
The means and standard deviations of protein and albumin levels in patients with SBP were higher than the corresponding rates in patients without SBP (P < 0.0001). Similar to the case with the other markers mentioned, the mean ± SD of LDH in patients with SBP was higher than the corresponding rate in patients without SBP (P < 0.0001). The means ± SD of both WBC and PMN counts were higher in patients with SBP (13587.6 ± 240.4 vs. 8029.4 ± 332.3, P = 0.005, and 3024.5 ± 265.4 vs. 1766.54 ± 435.3, P = 0.345, respectively).
The means ± SD of inflammatory markers such as CRP and ESR were significantly higher in patients with SBP in contrast to the rates for patients without SBP (P < 0.05) (Table 2).
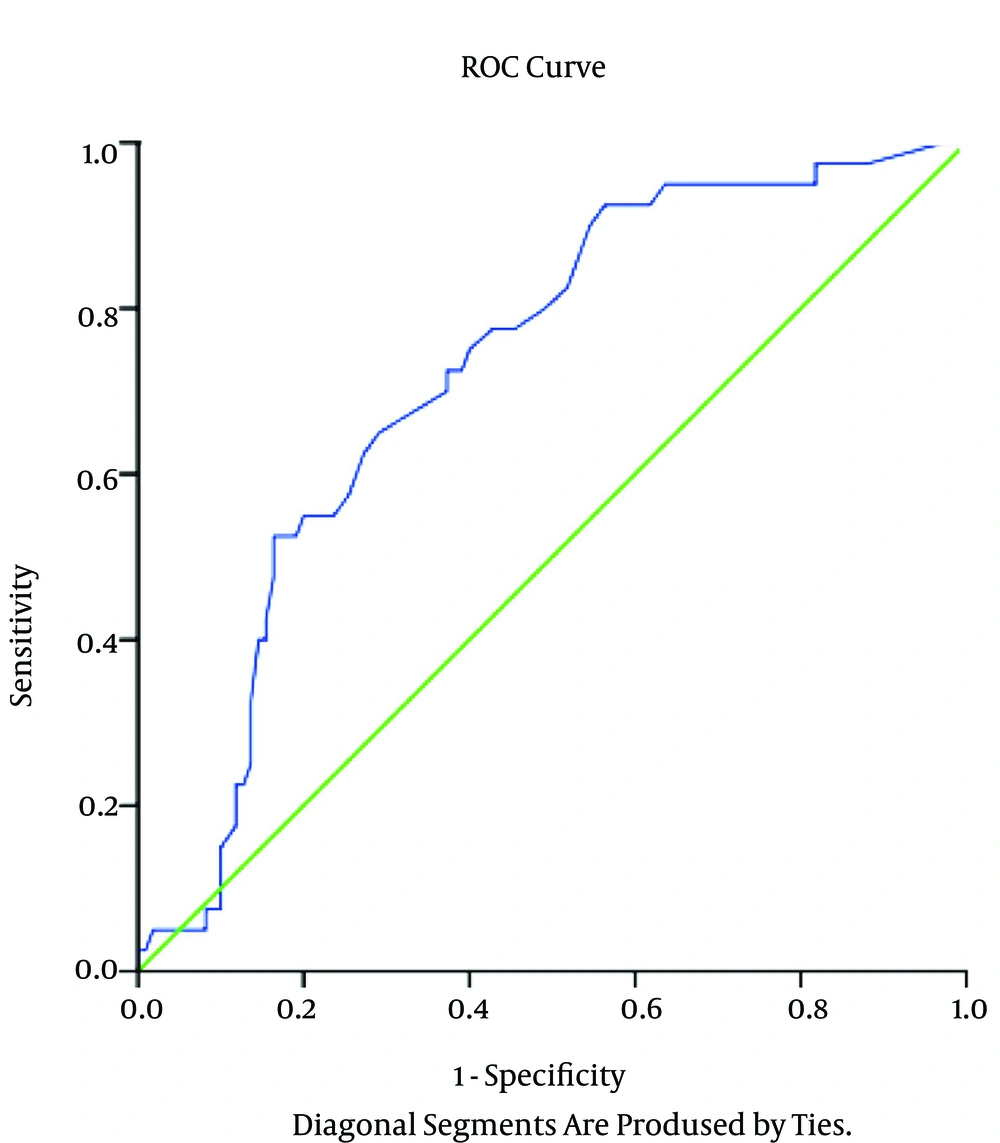
Table 3 and Figure 1 show the area under the curve and the predictive values of CRP for the diagnosis of SBP in children with liver disease. The percentages for sensitivity and specificity of the CRP marker for SBP in cirrhotic patients according to the PMN count of ascetic fluid were 69% and 90.25%, respectively (Table 3).
| Sen | Spe | PPV | NPV | Accuracy | AUC (CI) | P | |
|---|---|---|---|---|---|---|---|
| PMN ≥ 250/mm3 | 69.23 | 90.25 | 88.65 | 89.54 | 89.33 | 0.94 (0.90-0.96) | < 0.0001 |
| Positive Culture Ascetic | 88.43 | 84.32 | 85.48 | 90.32 | 85.63 | 0.85 (0.84-0.92) | < 0.0001 |
Abbreviations: AUC, area under curve; NPV, negative predictive value; PPV, positive predictive value; Sen, sensitivity; Spe, specificity.
aValues are expressed as %.
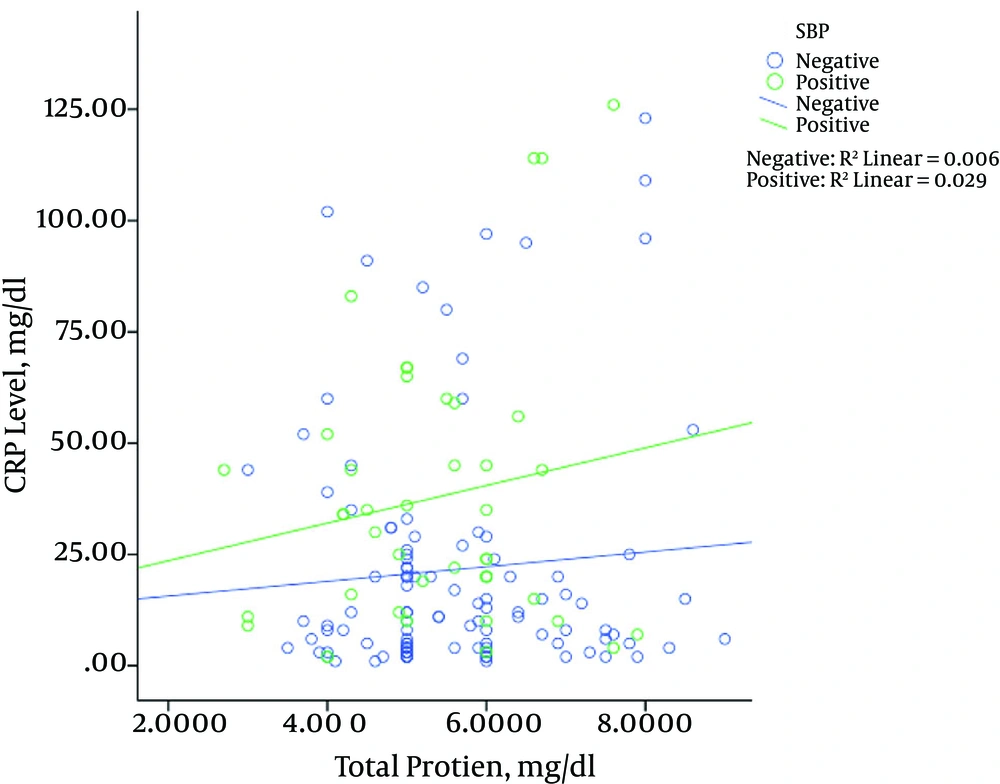
Figure 2 shows the relationship between the levels of CRP and total protein based on the presence (positive) or absence (negative) of SBP in cirrhotic patients. As shown in Figure 2, in both patients with and without SBP, the increased CRP levels were associated with increased total protein. However, this increase in patients with SBP showed higher severity than the increase in patients without SBP. Our results showed a significant correlation between total protein and CRP levels in children with and without SBP (P = 0.029, P = 0.006, respectively).
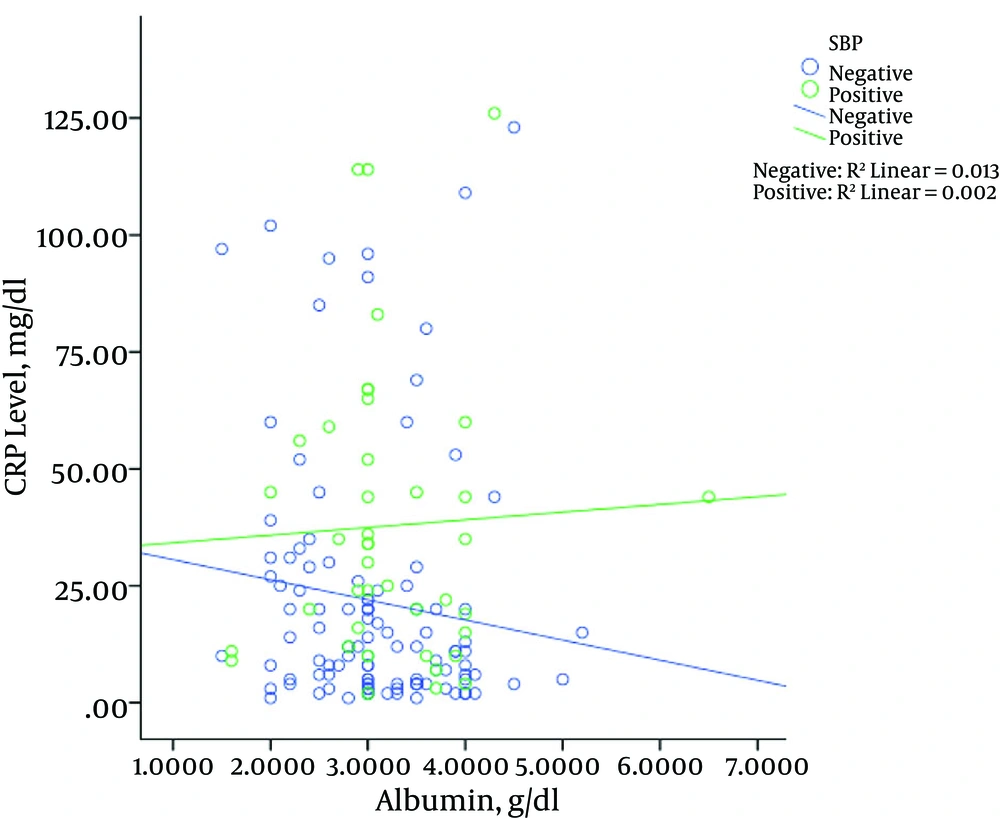
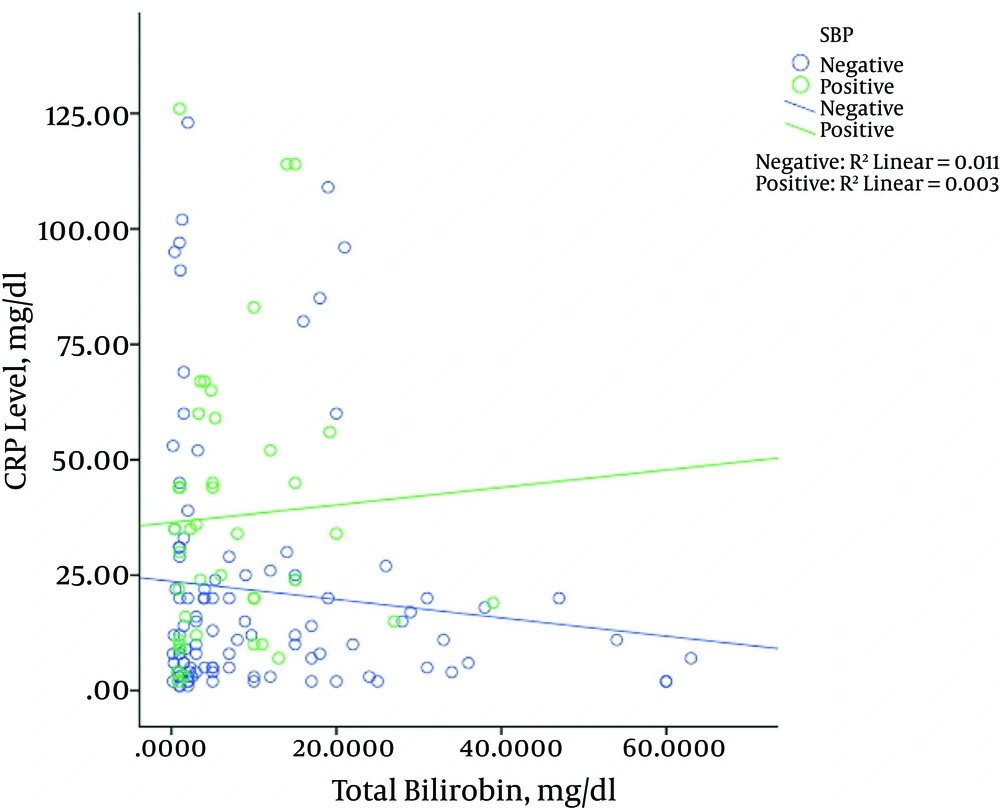
Figure 3 shows the relationship between albumin levels and CRP levels in patients with and without SBP. The results showed that in patients with SBP, increased levels of CRP were associated with increased albumin levels, which was significant (P = 0.002). However, this result was not observed in patients without SBP; the levels of CRP albumin in patients without SBP decreased significantly (P = 0.013). The same results were revealed for bilirubin (Figure 4), in that with increased CRP in patients with SBP, bilirubin levels increased significantly (P = 0.003), while in patients without SBP with increased levels of CRP, bilirubin levels decreased (P = 0.011). The relationship between the CRP levels with PT among the patients is shown in Figure 5. Increased protein level was associated with significantly increased PT (P = 0.006). However, in patients with SBP, PT did not change significantly according to the level of CRP changes (P = 0.697).
5. Discussion
SBP is a frequent and serious complication in cirrhotic patients that is associated with high in-hospital mortality rates of 20% - 30%, poor prognosis, and recurrence of over 70% in the first year of life. Early antibiotic treatment can reduce the mortality and morbidity of SBP, which requires performance and rapid diagnostic tests (26). Although extensive studies have been conducted for laboratory testing efficiency and various markers for the diagnosis of SBP (24, 26), the first report on the highly significant correlation between CRP and SBP was only published recently (24).
In the study by Preto-Zamperlini et al. on 90 children with chronic liver disease, Streptococcus pneumoniae was the most cultured organism (24). In our study, E. coli was the most frequently cultured organism. The serum WBC counts were significantly higher in the SBP group compared to the counts for patients without SBP. This finding was similar to the results of Preto-Zamperlini et al.’s study (24). Measurement of CRP in cirrhotic patients determined that serum CRP levels were significantly higher in patients with SBP compared to patients without SBP. In the study by Preto-Zamperlini et al., the CRP level was significantly higher in cases with SBP than in cases without SBP (24). In the study by Yuan et al., it was concluded that CRP is a better marker than WBC count for diagnosis of patients with chronic hepatitis B and SPB (27).
ESR was significantly higher in patients with SBP compared to patients without SBP. In another study by Viallon et al., the authors reported that CRP is significantly higher in adult cirrhotic patients with SBP compared to those that had sterile ascites fluid (28).
The serum total bilirubin levels were not significantly different between children with SBP and those without SBP. This finding was similar to those of other studies (24, 29).
In our study, albumin levels were significantly higher in the SBP group compared to the non-SBP group. In contrast to our study, albumin levels were significantly higher in the non-SBP group compared to SBP group in the study by Preto-Zamperlini et al. (24). In Vieira et al.’s study, no significant difference was found between SBP patients and those with non-SBP ascites (30). This difference may be due to the difference in sample size or the histories of albumin infusion.
In our study, no significant difference was found between the two groups in terms of PT and INR. This result is similar to the study by Vieira et al., who reported that these markers in patients with and without SBP were not significantly different (30). In contrast to the above study, a significant difference was found in the study by Preto-Zamperlini et al. (24). In another study, CRP was a significant indicator of infection in hospitalized children with cirrhosis (31). The difference between the severity of liver disease in these studies may be the cause of these differences.
The results of our study showed no significant difference regarding the age and sex of the patients with and without SBP.
As mentioned above, serum WBC count, ESR, and CRP may be good indicators of SBP in children with liver disease. However, further studies are required in this field because most of the available studies have been conducted on adult patients.
5.1. Limitation
The main limitation is the single-center focus of the study. Another limitation is the lack of serial measurement of CRP and the lack of finding a correlation between CRP and prognosis and recurrence of SBP. The small sample size of children with SBP is another limitation. Also, some selection bias could not be avoided.