1. Background
Hepatitis B virus (HBV) infections are a common global problem, despite effective vaccination and anti-viral therapies. HBV infection is the tenth leading cause of death in the world (1), with more than 350 million people affected by chronic hepatitis B (CHB) (2). CHB, along with liver cirrhosis and hepatocellular carcinoma (HCC), results in 0.5 - 1.2 million deaths each year (3). In Iran, 1.5 million people are infected with HBV, among whom 15% - 40% are predisposed to liver cirrhosis and/or HCC in the future (4). Genotype D is the most common genotype in Iran and is associated with the precore mutation and HBV e antigen (HBeAg)-negative CHB (5-7).
Many host and viral factors can affect the natural course of HBV infection. HBeAg, serum levels of HBV DNA, HBV genotype (C > B; D > A), co-infection with hepatitis C virus (HCV) or hepatitis D virus (HDV), as well as co-morbidities, including smoking and alcohol abuse, have been well-documented as risk predictors of cirrhosis and HCC in CHB patients (8). Identification of high-risk CHB carriers seems to be an acceptable strategy for prevention, or for the early diagnosis of liver cirrhosis, enabling further therapeutic management.
2. Objectives
The aim of this study was to determine the probable risk factors for developing cirrhosis in Iranian CHB patients.
3. Patients and Methods
3.1. Type of Study and Research Environment
This longitudinal study was performed from 1995 to 2014 on a group of 270 patients (aged 13 -78 years) who were diagnosed with HBsAg over a period of longer than six months. The cases were selected from the Tehran hepatitis center (THC), a referral center and the outpatient clinic of Baqiyatallah research center for gastroenterology and liver diseases, Baqiyatallah University of Medical Sciences, Tehran, Iran. Patients with positive results for HBsAg and who had completed at least five years of follow-up were included in this study. The exclusion criteria were cirrhosis as a first presentation, immunocompromised status, concurrent HCV, HIV infection or other liver diseases (autoimmune hepatitis, Wilson’s disease, hemochromatosis, and α-1-antitrypsin deficiency), or refusal to participate in the survey. Informed consent was obtained from all patients.
3.2. Follow-Up
The patients were assessed by one observer six months after their first visit, and then annually. The patients’ past medical histories were investigated for the presence of diabetes mellitus (DM), alcohol consumption, smoking, and opium addiction. In addition, a family history of liver disease, including carrier state of HBV, chronic HBV, cirrhosis, and HCC, were recorded. During each visit, we evaluated liver function tests, including serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as serum albumin, total bilirubin, prothrombin time (PT), fasting blood glucose (FBS), platelet count, and serological HBV markers. Viral markers (HBsAg, anti-HBs, HBeAg, and anti-HBe) were measured using the ELISA method (9). All of the included patients were assumed to be infected with HBV genotype D. Serum HBV DNA levels were quantitatively assessed with Cobas Amplicor and Cobas Taqman from Roche Diagnostics. The lower limit of detection is 200 copies/mL (38 IU/mL) for Amplicor and 30 copies (6 IU/mL) for Taqman. Validation and calibration of the laboratory equipment were approved by authorized sections.
The baseline presentation of the patients was determined according to the natural history of CHB infection, which is divided into 4 phases: 1) immune tolerance, 2) immune clearance (also referred to as immune active), 3) inactive carrier state, and 4) HBeAg-negative CHB (also known as reactivation) (10-12). The characteristics of the aforementioned phases are summarized in Table 1.
| Phase 1: Immune Tolerance | Phase 2: Immune Clearance | Phase 3: Inactive Carrier State | Phase 4: HBeAg-Negative CHB | |
|---|---|---|---|---|
| ALT | Normal | Elevated | Normal | Elevated |
| HBV DNA | Elevated, typically > 1 million IU/mL | Elevated ≥ 20,000 IU/mL | Low or undetectable < 2,000 IU/mL | Elevated ≥ 2,000 IU/mL |
| HBeAg | Positive | Positive | Negative | Negative |
| Liver histology | Normal or mild hepatitis | Moderate or severe necroinflammation and fibrosis | Normal or mild inflammation | Moderate to severe inflammation and fibrosis ± cirrhosis |
The diagnosis of liver cirrhosis was based on ultrasonographic findings supported by liver histology, thrombocytopenia, or direct observation of esophageal varices on upper gastrointestinal endoscopy. For HCC, the diagnosis was made when a patient met one of the following two criteria: a non-invasive criterion using multidetector CT scan/dynamic contrast-enhanced MRI showing typical hypervascularity in the arterial phase with washout in the portal vein or delayed phases, or a pathologic biopsy compatible with malignancy (13, 14).
Eligible patients were treated according to the latest guidelines for HBV treatment. The study protocol was approved by the ethics committee of Baqiyatallah University of Medical Sciences (Code 340/5/5904 number 91 on 11/15/14).
3.3. Data Analysis
Univariate and multiple logistic regression analyses were performed, yielding regression coefficients, odds ratios (ORs), and P values. The response or outcome variable of the analyses was the presence/absence of liver cirrhosis. Univariate analyses were initially performed to identify potential determinants of outcome of all variables assumed to be associated with liver cirrhosis. Laboratory parameters were categorized into two different groups based on defined normal ranges. Chi-square was used to determine the statistical significance of the relationship between the independent and dependent variables (cirrhotic and non-cirrhotic). After exclusion of those variables with no prognostic significance, appropriate potential variables were determined for multivariate analysis. Backward stepwise logistic regression was then recruited to identify predictive factors (with α > 0.05 taken as a level of entry and exclusion) that were correlated with our outcome. The data were statistically analyzed using the IBM SPSS statistics software, version 22.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant.
4. Results
4.1. Patient Details
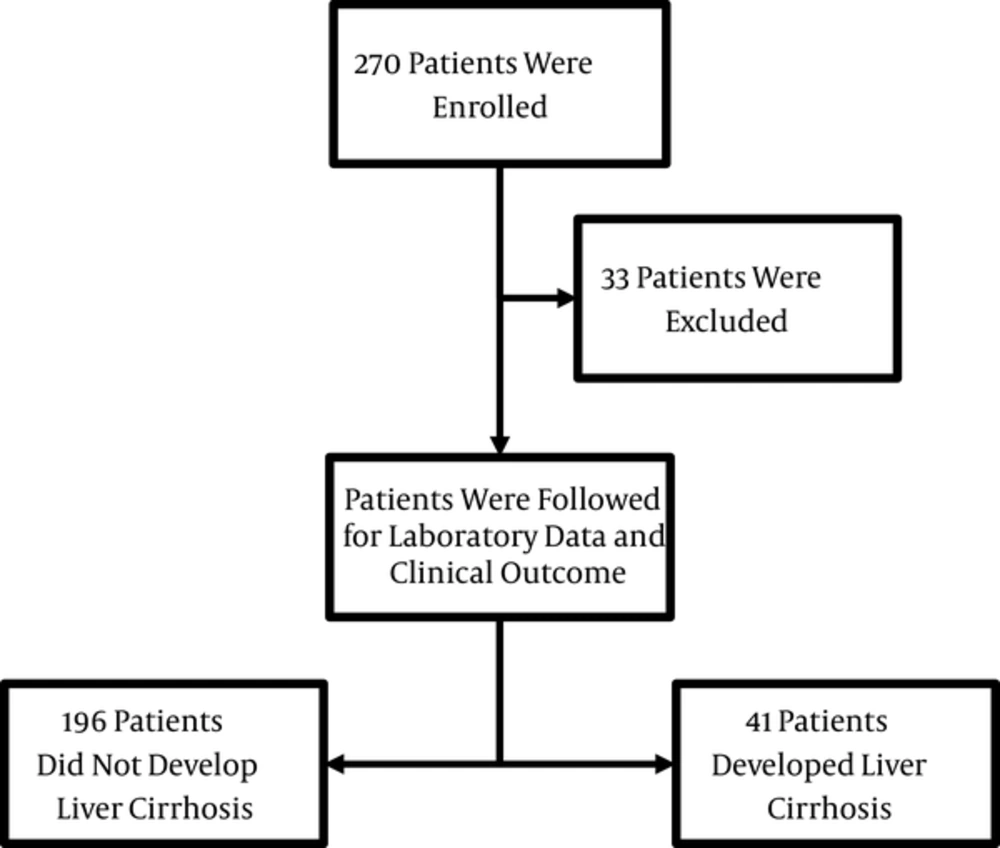
After the exclusion of 33 patients, a total of 237 CHB patients were recruited, of whom 41 (17.3%) developed liver cirrhosis (Figure 1). The median and mean duration of follow-up of the cirrhotic patients were 12 years (range 5 - 18) and 11.4 years (SD = 2.6), respectively. The incidence rates of cirrhosis and HCC were 2.82/100 and 0.75/100 person-years, respectively. A positive family history of HBV infection in at least one first-degree relative was present in 54.9% (n = 130) of the patients, and the majority of these did not present with liver cirrhosis (P = 0.037).
The baseline presentations of the patients are shown in Table 1. Forty-two, 45, 44, and 106 patients initially presented in phases 1 through 4, respectively, of CHB.
4.2. Factors Associated With the Development of Liver Cirrhosis
Univariate analyses determined 9 out of 17 factors as significant predictors of liver cirrhosis in CHB patients (Tables 2 and 3). Liver cirrhosis was significantly higher in older patients (≥ 50 years) and diabetic patients (P < 0.001 and P = 0.001, respectively). There was a significant association between liver cirrhosis and HDV infection (P < 0.001) and HCC (P < 0.001). The prevalence of HDV in CHB patients was 10.1%. Older CHB patients had significantly higher rates of HDV infection (P < 0.001) and HBeAg negativity (P < 0.001).
| Parameters | Overall (n = 237) | Cirrhotic (n = 41) | Non-Cirrhotic (n = 196) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, y | 10.892 (4.096 - 28.969) | < 0.001a | |||
| < 45 | 123 | 5 | 118 | ||
| ≥ 45 | 114 | 36 | 78 | ||
| Sex (M : Fb) | 171:66 | 30:11 | 141:55 | 1.064 (0.499 - 2.27) | 0.873 |
| Follow-Up, y | 0.06 | ||||
| Mean ± SD | 10.6 ± 2.9 | 11.4 ± 2.6 | 10.5 ± 2.9 | ||
| Median (range) | 11 (5 - 19) | 12 (5 - 18) | 10 (5 - 19) | ||
| Smokerc | 21 | 5 | 16 | 1.562 (0.538 - 4.537) | 0.412 |
| DMc | 46 | 16 | 30 | 3.541 (1.693 - 7.409) | 0.001a |
| Alcoholc | 21 | 3 | 18 | 1.281 (0.359 - 4.568) | 0.703 |
| Steatosisc | 45 | 9 | 36 | 1.25 (0.549 - 2.847) | 0.595 |
| Negative HBeAgc | 87 | 5 | 82 | 5.179 (1.949-13.765) | 0.001a |
| HDVc | 24 | 15 | 9 | 11.987 (4.765 - 30.158) | < 0.001a |
| HCCc | 12 | 8 | 4 | 11.636 (3.315 - 40.85) | < 0.001a |
| Positive family history | |||||
| HBV | 130 | 16 | 114 | - | - |
| Cirrhosis | 25 | 6 | 19 | - | - |
| HCC | 3 | 0 | 3 | - | - |
aStatistically significant (P value < 0.05).
bReference level.
cReference levels are non-smokers, patients without DM, alcoholics, patients with steatosis, seronegative HBeAg status, seronegative HDV status, and non-HCC patients, respectively.
| Parametersa | Overall (n = 237) | Cirrhotic (n = 41) | Non-Cirrhotic (n = 196) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Albumin, g/Lb | 1.037 (0.284 - 3.788) | 0.956 | |||
| < 35c | 17 | 3 | 14 | ||
| ≥ 35 | 209 | 38 | 171 | ||
| AST, U/L | 3.77 (1.816 - 7.825) | < 0.001d | |||
| < 40c | 189 | 24 | 165 | ||
| ≥ 40 | 48 | 17 | 31 | ||
| ALT, U/L | 1.251 (0.609 - 2.567) | 0.542 | |||
| < 45 | 152 | 28 | 124 | 0.542 | |
| ≥ 45c | 85 | 13 | 72 | ||
| Total bilirubin, μmol/L | 3.227 (1.405 - 6.275) | 0.006d | |||
| < 15c | 206 | 30 | 176 | ||
| ≥ 15 | 31 | 11 | 20 | ||
| PT, s | 2.6 (0.878 - 7.699) | 0.085 | |||
| < 13c | 47 | 4 | 43 | ||
| ≥ 13 | 190 | 37 | 153 | ||
| Platelet count, × 109/L | 10.216 (4.821 - 21.651) | < 0.001d | |||
| < 150 | 51 | 25 | 26 | ||
| ≥ 150c | 186 | 16 | 170 | ||
| FBS (mg/dL)e | 1.623 (0.42 - 6.275) | 0.483 | |||
| <126c | 223 | 38 | 185 | ||
| ≥126 | 12 | 3 | 9 | ||
| HBV DNA level, IU/mLf | 4.372 (2.136 - 8.952) | < 0.001d | |||
| < 2,000c | 189 | 20 | 169 | ||
| ≥ 2,000 | 44 | 19 | 25 |
aLaboratory parameters were categorized into two different groups based on defined normal ranges.
bData were not available for 11 patients.
cReference level.
dStatistically significant (P value < 0.05).
eData were not available for 2 patients.
fData were not available for 4 patients.
The laboratory data of the patients were monitored during follow-up. Patients who developed liver cirrhosis had significantly higher median AST levels and higher median bilirubin levels during follow-up compared to non-cirrhotic patients. There was a meaningful correlation between lower median platelet levels and higher median HBV DNA levels in the sera of CHB patients, and the development of liver cirrhosis. P values are presented in (Table 1).
There was no association between liver cirrhosis and sex, smoking, alcohol consumption, or liver steatosis, or with serum albumin, ALT, PT, or FBS levels.
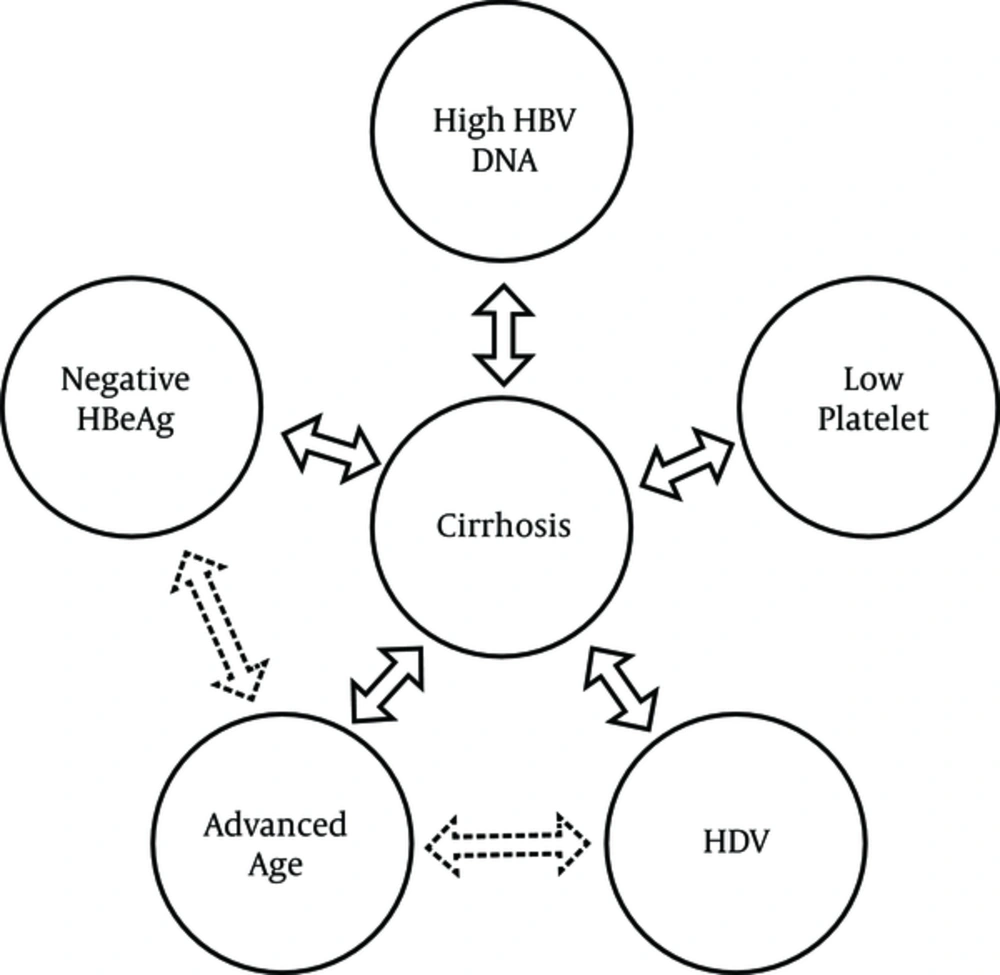
In this study, an age of ≥ 45 years, HDV positivity, HBeAg negativity, a platelet count of < 150 (× 109)/L, and an HBV DNA level of ≥ 2,000 IU/mL were identified as significant independent predictors of liver cirrhosis (Table 4). Other factors implicated in the univariate analysis did not reach statistical significance in multiple logistic regression analyses.
| Variable | β | SE*(β) | OR | CI95 | P Value |
|---|---|---|---|---|---|
| Age ( ≥ 45 vs. < 45 years) | 1.624 | 0.58 | 5.073 | 1.629 - 15.799 | 0.005 |
| HDV (positive vs. negative) | 2.487 | 0.674 | 12.026 | 3.209 - 45.068 | < 0.001 |
| HBeAg (negative vs. positive) | 1.352 | 0.666 | 3.867 | 1.049 - 14.251 | 0.042 |
| Platelet count (< 150 vs. ≥ 150 ×109/L) | 1.066 | 0.503 | 2.903 | 1.083 - 7.777 | 0.034 |
| HBV DNA level ( ≥ 2,000 vs. < 2,000 IU/mL) | 2.255 | 0.516 | 9.538 | 3.471 - 26.211 | < 0.001 |
Abbreviations: CI95, 95% confidence interval; OR, odds ratio; and SE, standard error.
aA total of 233 patients were used for the multivariate logistic analysis.
5. Discussion
During a mean period of ten years of follow-up, 237 CHB patients were monitored for probable predictors of liver cirrhosis. Most of the patients were male and under 45 years old (n = 171 and n = 123, respectively). The incidence rates of cirrhosis and HCC in CHB patients were 2.82/100 and 0.75/100 person-years, respectively, which is comparable to the similar data from Iran and other countries (8, 14, 15). HBV genotype D is the most predominant genotype in Iranian subjects suffering from CHB (6). Genotype D may be associated with higher rates of hepatoma, post-transplant recurrence, and mortality compared with genotype A (16).
Our results suggest that five different parameters represent significant risk factors for the development of liver cirrhosis in CHB patients. In the last few decades, the mean age at infection with HBV has increased in Iran as a result of the establishment of a national hepatitis B vaccination program in 1993 and improved immunity of young children against HBV infection (17, 18). Older CHB patients are more likely to develop cirrhosis during follow-up, as seen in similar studies from East Asian and European countries (15, 19-23).
In this study, HDV prevalence was 10.1% in CHB patients, which is in the range of the results of other studies from our country and around the world (24, 25). HDV positivity is the most significant risk factor for the incidence of cirrhosis in CHB patients. Co-infection with HDV worsens the CHB patient’s condition. HDV-infected patients are more likely to present with serious complications compared to HBV patients without HDV, and the relative risk of developing cirrhosis in HDV-infected patients seems to be doubled (8, 26). The later development of liver cirrhosis in HDV-infected patients decreases the probability of survival to 49% and 40% at 5 and 10 years, respectively (27). It is important to screen CHB patients for HDV for better management of concurrent infections, and for close observation for the early diagnosis of subsequent complications.
We demonstrated that cirrhosis was four times more common in HBeAg-negative patients. East Asian countries show the same pattern for the development of cirrhosis in HBeAg-negative patients (28-31). HBeAg-negative patients are usually older than those who are HBeAg-positive, which may be due to the fact that HBeAg-negative chronic hepatitis represents a late stage in the natural course of CHB infection, and HBeAg-negative patients have a longer duration of liver disease (8, 32, 33). In addition, many mutations (such as precore G1896A stop codon mutation or double nucleotide mutations [A1762T/G1764A] in basal core promoter) are associated with the HBeAg-negative CHB phase. These mutations, alone or in combination, not only can result in loss of HBeAg synthesis but can also promote progressive liver disease, including cirrhosis (34). However, the overall incidence rates of cirrhosis in HBeAg-negative patients in western countries are higher than in Asian countries (8, 35). The inclusion of a large proportion of HBeAg-positive carriers presenting in the immune tolerant phase of CHB infection can provide a valuable clue to why East Asian studies demonstrate lower rates of cirrhosis in HBeAg-negative patients (8). Accordingly, substantial progression of fibrosis in the liver histology after transition from the immune tolerant phase to the immune clearance phase occurs as a result of immune-mediated liver damage (36). Of note, genotype D is associated with HBeAg-negative disease and a more severe disease course compared to other genotypes. HBeAg seroconversion occurs at higher rates and at younger ages among patients with genotype D compared to other genotypes (5, 7, 37). Despite the fact that they are independent risk factors for the development of liver cirrhosis, advanced age and negative HBeAg status were strongly correlated with extensive liver fibrosis in the present study (Figure 2).
In the present study, we were able to demonstrate that thrombocytopenia had a substantial impact on the development of cirrhosis in CHB patients. Despite the fact that platelet sequestration in the spleen and decreased production of thrombopoietin in the liver contributes to thrombocytopenia in liver cirrhosis, experimental data show that thrombocytopenia per se might be implicated in the progression of liver fibrosis (38-40). Mohamadnejad et al. also reported that a low platelet count was an independent risk factor for noticeable fibrosis in CHB patients (41). This implies a two-sided correlation between thrombocytopenia and progression to liver cirrhosis.
The current study revealed that higher serum HBV DNA levels (≥ 2000 IU/mL) strongly correlated with progression to liver cirrhosis. Iloeje et al. have shown that with increasing HBV DNA levels in CHB patients, the risk of cirrhosis increases (20). In a similar study by Wong et al. (42), the odds ratios for cirrhosis began to increase significantly when serum HBV DNA was > 2,000 IU/mL in HBeAg-negative CHB patients. Despite the fact that serum HBV DNA levels had no significant correlation with liver histology, an HBV DNA level of 4,000 IU/mL could predict considerable liver fibrosis with a sensitivity of 82% and specificity of 70% in HBeAg-negative patients (41, 43, 44). Therefore, close monitoring of the serum HBV DNA level is recommended due to the risk of CHB progression to liver cirrhosis with elevated levels (45). On the other hand, genotype D patients had the lowest chance of sustained response to interferon therapy, regardless of serum HBV DNA or ALT levels (46).
The strong points of this study were the 10 years of patient follow-up, the relatively appropriate sample size, and complete laboratory data. However, a limitation of the present investigation was that we studied the patients at a single center. In addition, the univariate analyses identified 8 out of 17 factors as unsuitable predictors of liver cirrhosis in CHB patients. This may be due to actual insignificant differences or an insufficient sample size.
In conclusion, significant predictors of liver cirrhosis, such as an age of ≥ 45 years, HDV positivity, HBeAg negativity, a platelet count of < 150 (× 109)/L, and an HBV DNA level of ≥ 2,000 IU/mL, may provide valid information for further diagnostic and therapeutic decisions in CHB patients. These data are simple to gather, easy to measure, and cost-effective for the prediction of liver cirrhosis.